Quest for the right Drug
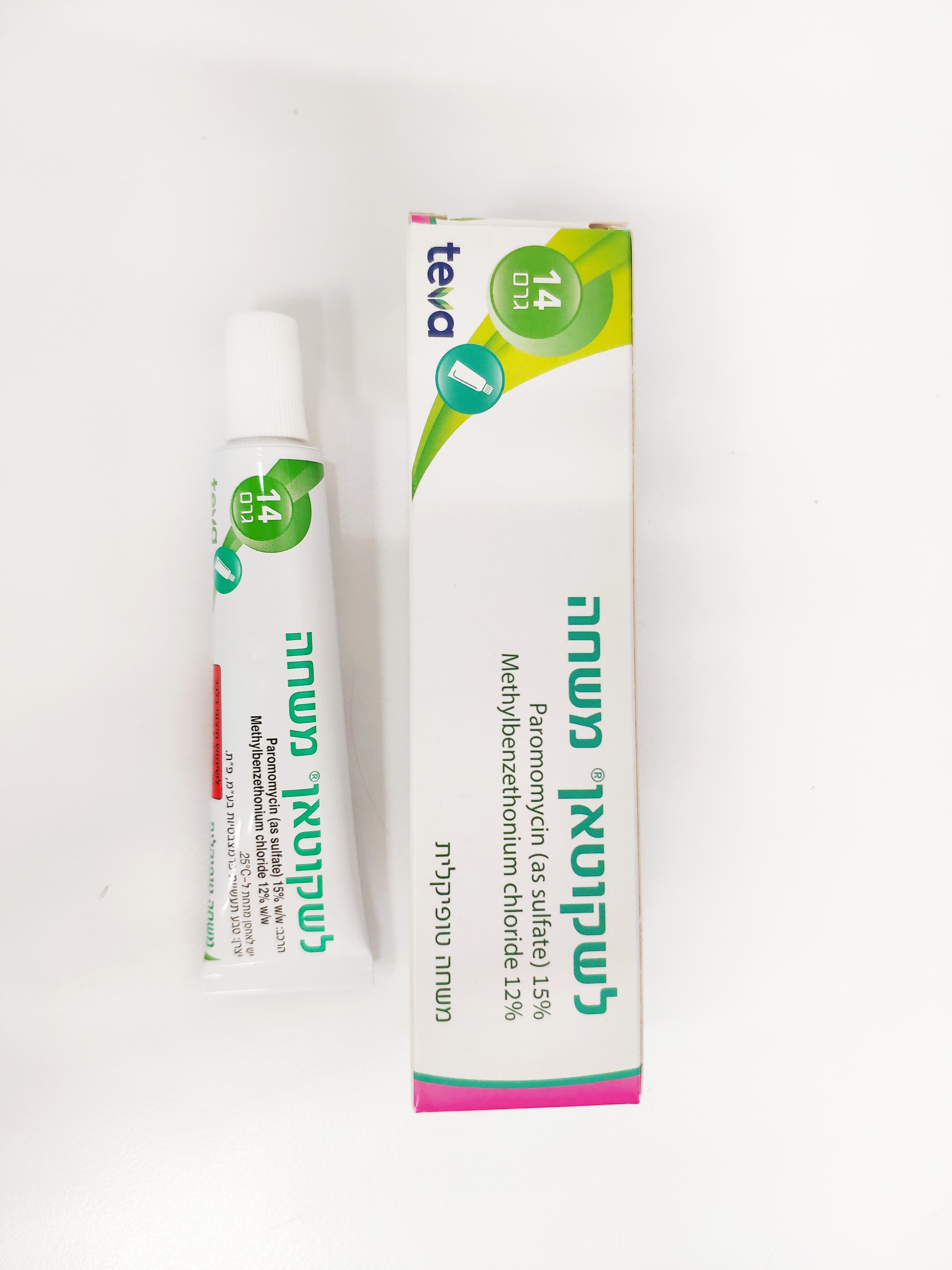
לשקוטאן משחה LESHCUTAN OINTMENT (METHYLBENZETHONIUM CHLORIDE, PAROMOMYCIN AS SULFATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
חיצוני : TOPICAL
צורת מינון:
משחה : OINTMENT
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Action Paromomycin is an aminoglycoside antibiotic produced by the growth of certain strains of Streptomyces rimosus var paromomycinus. It has an antimicrobial Leshcutan Ointment FW Minor updates 01-2022 spectrum similar to that of neomycin sulfate. There is a cross-resistance between paromomycin, neomycin, kanamycin and framycetin. Paromomycin administered orally has for many years been used for the treatment of both acute and chronic intestinal amebiasis, in the suppression of intestinal flora, both preoperatively and in the management of hepatic coma. Methylbenzethonium chloride is a quaternary ammonium compound with antimicrobial activity. It is widely used as a topical anti-infective and also as a preservative and cationic surfactant in a variety of pharmaceutical and cosmetic preparations. Pharmacodynamics Paromomycin, like all the aminoglycoside antibiotics, inhibits protein biosynthesis in sensitive organisms. Electron microscopy studies indicated that the main organelles of promastigotes and amastigotes affected by Leshcutan are the mitochondria by methylbenzethonium chloride and the reticular system by paromomycin. Paromomycin was studied in vitro on Leishmania major amastigotes cultured in C3H/He mouse peritoneal exudate cells. The therapeutic activity, expressed as the minimum effective concentration which reduced the parasite survival index by 50% after 48 hours exposure to paromomycin, was 10 g/ml. Methylbenzethonium chloride was reported to decrease the growth of Leishmania major promastigotes and amastigotes in vitro. The effect of different concentrations of paromomycin with various percentages of methylbenzethonium chloride on the development of Leishmania major in BALB/c and C3H/He mice was studied. Ointments containing either paromomycin alone or methylbenzethonium chloride alone had only a partial effect on the parasites. The in vivo efficacy of Leshcutan containing 15% paromomycin and 12% methylbenzethonium chloride against experimentally-induced cutaneous leishmaniasis in BALB/c and C3H/He mice demonstrated a 100% cure rate in mice inoculated with L. major and L. mexicana.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Paromomycin is poorly absorbed after oral therapy. However, following intramuscular injection of single and multiple injections of paromomycin, peak serum levels occur within the first 2 hours, followed by a rapid fall of drug concentration in the serum and the appearance of high concentrations of the drug in the urine. The half-life of serum antibiotic activity is approximately 5 hours. No local or systemic toxic reactions attributable to the drug were observed, nor was there any evidence of accumulation of the drug in the body in the presence of normal renal function. In the presence of an abnormal kidney, excretion of paromomycin may be hindered, therefore potential nephrotoxic symptoms might occur. Intramuscular injections of paromomycin do not seem to penetrate the blood-brain barrier. The kinetics of paromomycin following topical application was examined using the microbiological inhibition zone method. Sera were collected from patients on the last day of a 10-day course of treatment at 1, 4 and 24 hours after termination of treatment with 15% paromomycin and 12% methylbenzethonium chloride. Bioassay using Staphylococcus epidermitidis, a test sensitive to 0.1 g/ml of paromomycin, failed to detect the drug in the sera of treated patients at any time point tested. Leshcutan Ointment FW Minor updates 01-2022

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/2000
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
