Quest for the right Drug
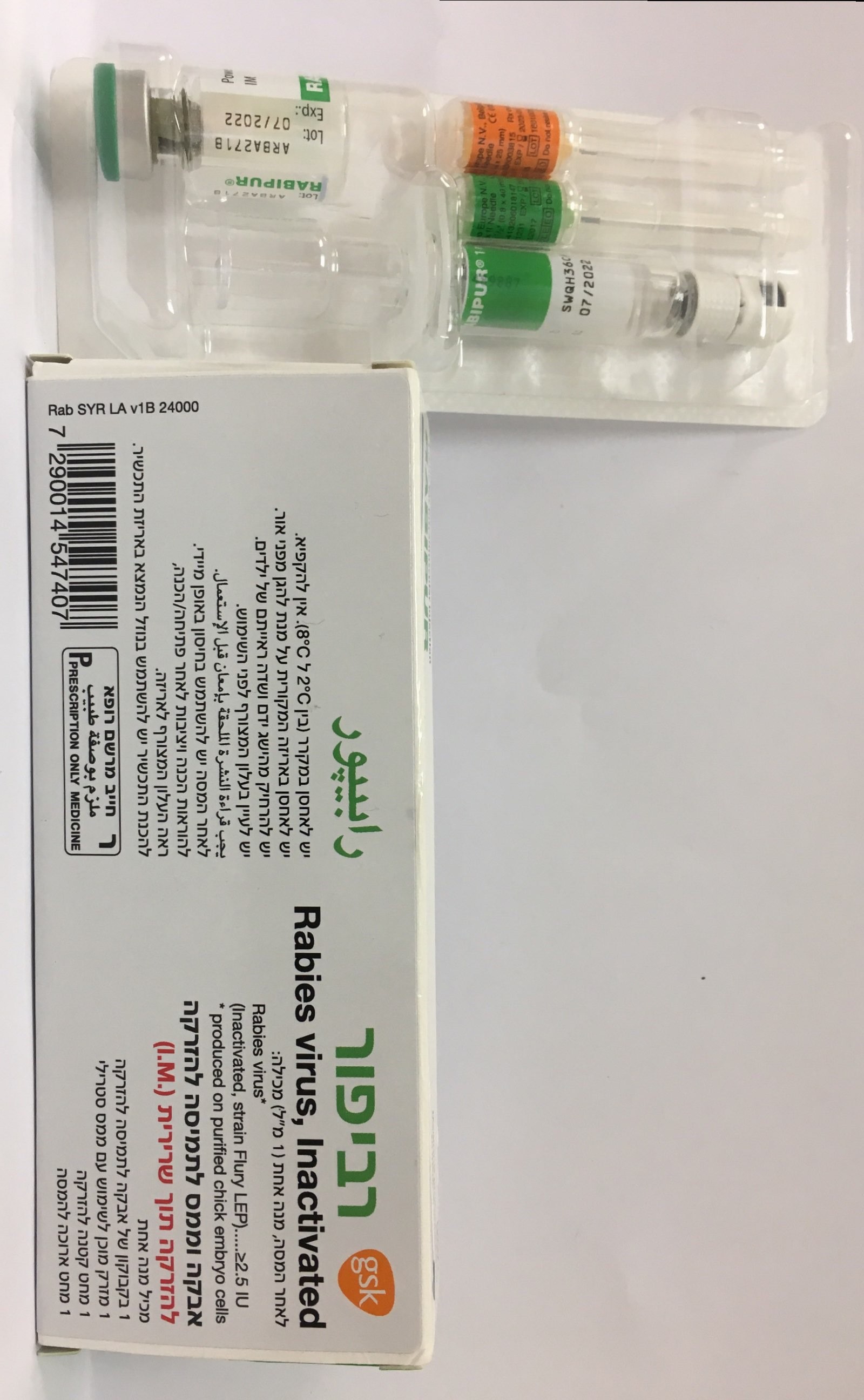
רביפור RABIPUR (RABIES, INACTIVATED, WHOLE VIRUS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
אבקה וממס להכנת תמיסה להזרקה : POWDER AND SOLVENT FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Powder: TRIS (hydroxymethyl)-aminomethane Sodium chloride Titriplex III (Disodium edetate) Potassium-L-glutamate Polygeline (Haemaccel) Sucrose Solvent: Water for injection 6.2 Incompatibilities In the absence of compatibility studies, Rabipur must not be mixed in the same syringe with other medicinal products. 6.3 Shelf life The expiry date of the vaccine is indicated on the label and packaging. After reconstitution the vaccine is to be used immediately. 6.4 Special precautions for storage Store in a refrigerator (2°C - 8°C). Do not freeze. Keep the vial and the syringe in the outer carton in order to protect from light. For storage conditions after reconstitution of the medicinal product, see section 6.3 The vaccine may not be used after the expiration date given on package and container. 6.5 Nature and contents of container Package with: 1 vial (type I glass) of freeze-dried vaccine with stopper (chlorobutyl) 1 disposable pre-filled syringe (type I glass) of Sterile Diluent for reconstitution (1 mL) with plunger-stopper (bromobutyl) without needle and with a tip-cap (bromobutyl). 1 small orange Rabipur_Vial_PFS_SPC_V4.1_Update_09-2019 Page 9 of 12 needle for injection (25 gauge, 25 mm) and one long green needle for reconstitution (21 gauge, 40 mm) 6.6 Special precautions for disposal and other handling Instruction for Use of Rabipur disposable pre-filled syringe: Pre-filled syringe Step 1: With one hand, hold the syringe (E) with the cap pointing upward. Be sure to hold the syringe by the white textured holding ring (D). Step 2: With the other hand, grasp the cap (A) and firmly rock it back and forth to break its connection to the holding ring (D). Do not twist or turn the cap. Step 3: Lift up to remove the cap (A) and the attached gray tip cap (B). Be careful not to touch the sterile syringe tip (C). Needle application (these instructions apply to both the green and the orange needles): Rabipur_Vial_PFS_SPC_V4.1_Update_09-2019 Page 10 of 12 Step 1: Twist to remove the cap (H) from the green reconstitution needle. Do not remove the plastic cover (G). This needle is the longer of the two needles. Step 2: With one hand, firmly hold syringe (E) by white textured holding ring (D). With your other hand, insert needle (F) and twist clockwise until it locks into place. Once needle is locked, remove its plastic cover (G). The syringe is now ready for use. Instructions for reconstituting Rabipur with the use of pre-filled syringe: The vaccine should be visually inspected both before and after reconstitution for any foreign particulate matter and or change in physical appearance. The vaccine must not be used if any change in the appearance of the vaccine has taken place. The reconstituted vaccine is clear to slightly opalescent and colourless to slightly pink. The powder for solution should be reconstituted using the solvent for solution supplied and carefully agitated prior to injection. The reconstituted vaccine should be used immediately. During manufacturing, the vial is sealed under vacuum. Therefore to prevent problems in withdrawing the reconstituted vaccine from the vial after reconstitution of the vaccine, it is recommended to unscrew the syringe from the needle to eliminate the negative pressure. After that, the vaccine can be easily withdrawn from the vial. It is not recommended to induce excess pressure, since over-pressurization will create the problems in withdrawing the proper amount of the vaccine. After completing the reconstitution of the vaccine, remove the cap from the orange administration needle (as explained in step 1 for the green needle) and replace the green reconstitution needle with the orange administration needle or other appropriate needle. Any unused vaccine or waste material should be disposed of in accordance with local requirements.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
