Quest for the right Drug
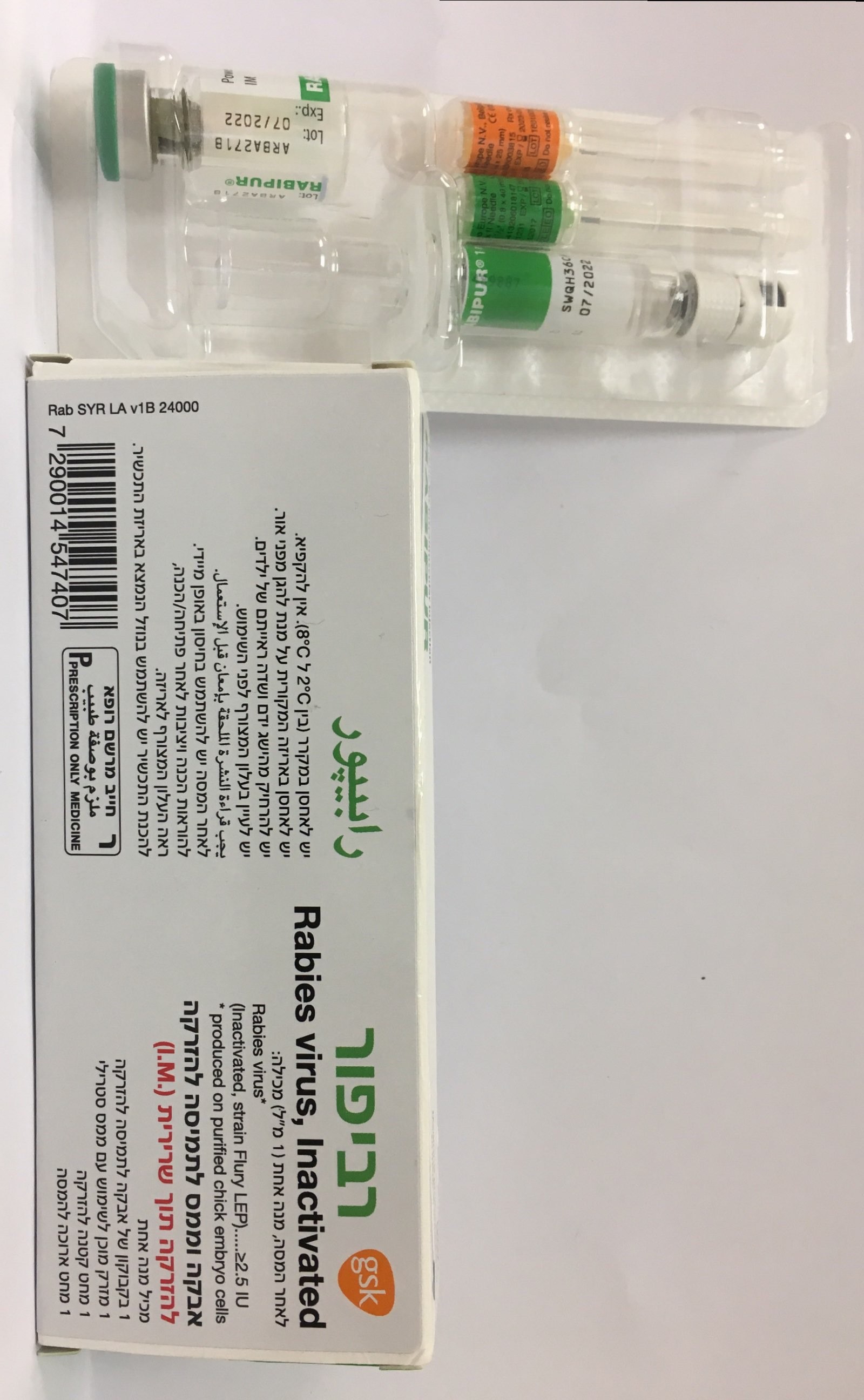
רביפור RABIPUR (RABIES, INACTIVATED, WHOLE VIRUS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
אבקה וממס להכנת תמיסה להזרקה : POWDER AND SOLVENT FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: ATC code: J07B G01 Mechanism of action Rabipur induces stimulation of lymphocytes and antibody-secreting plasmocytes resulting in production of RVNAs. Clinical efficacy and safety Pre-exposure prophylaxis In clinical trials with previously unimmunised subjects almost all subjects achieve an adequate immune response (RVNAs ≥ 0.5 IU/ml) 3 to 4 weeks after the end of a primary series of three injections of Rabipur when given according to the recommended schedule by the intramuscular route. Persistence of adequate immune response (RVNAs ≥ 0.5 IU/ml) for up to 2 years after primary immunization with Rabipur without booster doses has been found in clinical studies. As antibody concentrations slowly decrease, booster doses may be required to maintain antibody levels above 0.5 IU/ml. The timing of booster doses after primary vaccination with rapid regimen or after concomitant vaccination has not yet been established. Due to a faster decline in immune response compared with the conventional schedule a shorter interval between primary vaccination and booster administration may be needed compared with the conventional vaccine schedule. (see section 4.2). In a clinical trial, a booster dose of Rabipur administered 1 year after primary immunisation elicited a 10-fold or higher increase in Geometric Mean Concentrations (GMCs) by day 30. It has also been demonstrated that individuals who had previously been immunised with Human Diploid Cell Vaccine (HDCV) developed a rapid anamnestic response when boosted with Rabipur. Post-exposure prophylaxis In clinical studies Rabipur elicited adequate neutralising antibodies (≥ 0.5 IU/ml) in almost all subjects by day 14 or 30, when administered according to the 5- dose (day 0, 3, 7, 14, 28; 1.0 ml each, intramuscular) Essen regimen or 4-dose (day 0 [2 doses], 7, 21; 1.0 ml each, intramuscular) Zagreb regimen. Concomitant administration of Human Rabies Immunoglobulin with the first dose of rabies vaccine caused a slight decrease in GMCs (Essen regimen). However, this was not considered to be clinically relevant. Rabipur_Vial_PFS_SPC_V4.1_Update_09-2019 Page 8 of 12
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Not applicable

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
