Quest for the right Drug
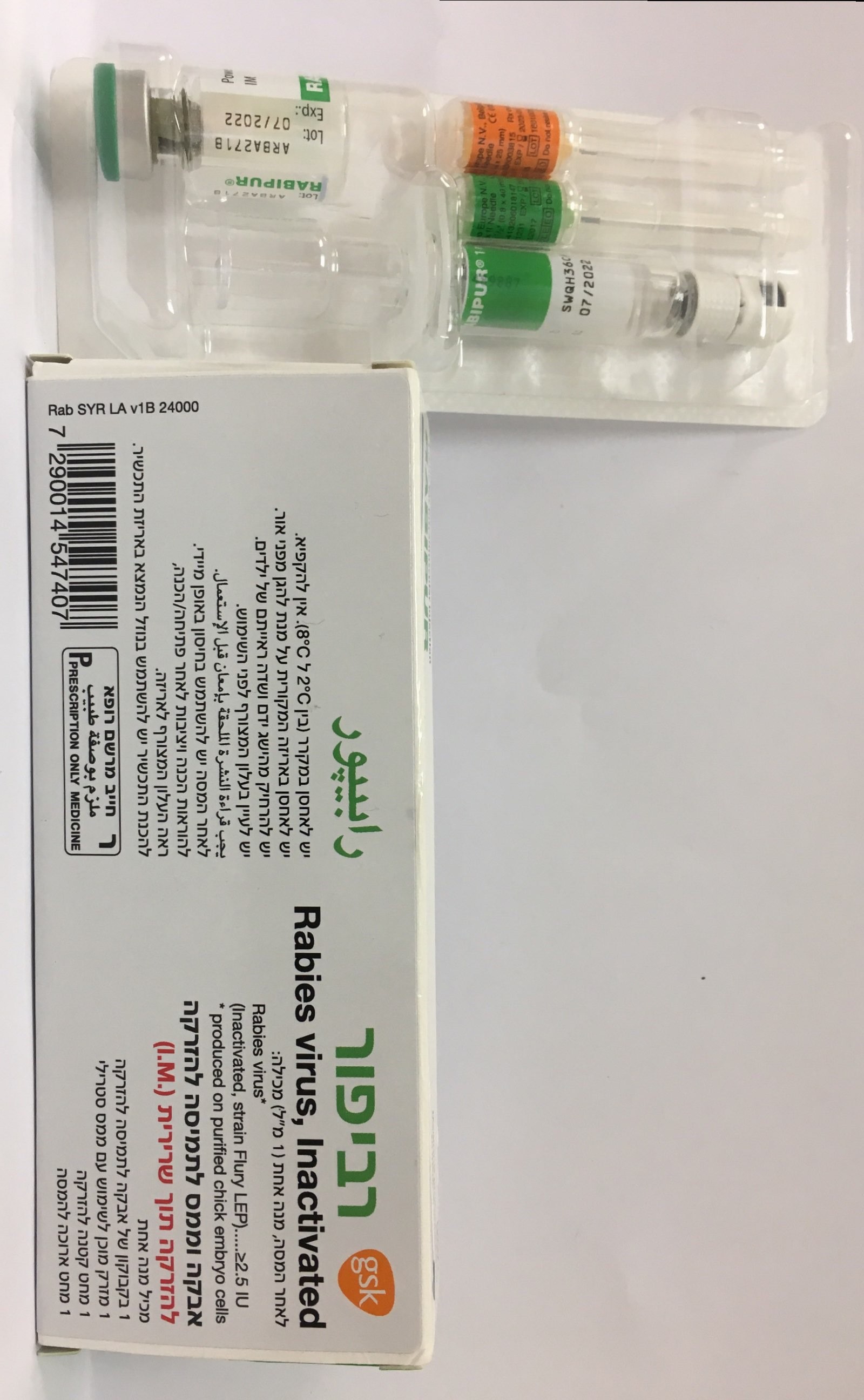
רביפור RABIPUR (RABIES, INACTIVATED, WHOLE VIRUS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
אבקה וממס להכנת תמיסה להזרקה : POWDER AND SOLVENT FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use A protective immune response may not be elicited in all vaccinees. In case of acute diseases requiring treatment, patients should not be vaccinated until at least 2 weeks after recovery. The presence of a minor infection should not result in the deferral of vaccination. Hypersensitivity reactions (PEP only) Anaphylactic reactions including anaphylactic shock have occurred following Rabipur vaccination. As with all injectable vaccines, appropriate medical treatment and supervision should always be readily available in case of a rare anaphylactic event following the administration of the vaccine. Rabipur contains the excipient polygeline, residues of chicken proteins (e.g., ovalbumin), human serum albumin, and may contain traces of antibiotics (see section 2). In instances in which individuals have developed clinical symptoms of anaphylaxis such as generalized urticaria, upper Rabipur_Vial_PFS_SPC_V4.1_Update_09-2019 Page 4 of 12 airway (lip, tongue, throat, laryngeal or epiglottal) oedema, laryngeal spasm or bronchospasm, hypotension or shock, following exposure to any of these substances, the vaccination should only be administered by personnel with the capability and facilities to manage anaphylaxis post-vaccination Central nervous system effects Encephalitis and Guillain-Barré syndrome have been temporally associated with the use of Rabipur (see also section 4.8). A patient's risk of developing rabies must be carefully considered, before deciding to discontinue immunization. Route of administration Rabies vaccine must not be given by intra-gluteal injection or subcutaneously, as the induction of an adequate immune response may be less reliable. Unintentional intravascular injection may result in systemic reactions, including shock. Do not inject intravascularly. Anxiety-related reactions Anxiety-related reactions, including vasovagal reactions (syncope), hyperventilation or stress- related reactions, may occur in association with vaccination as a psychogenic response to the needle injection (see section 4.8). It is important that procedures are in place to avoid injury from fainting.
Effects on Driving
4.7 Effects on ability to drive and use machines Some of the adverse effects described in section 4.8, may affect the ability to drive and use machines.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
