Quest for the right Drug
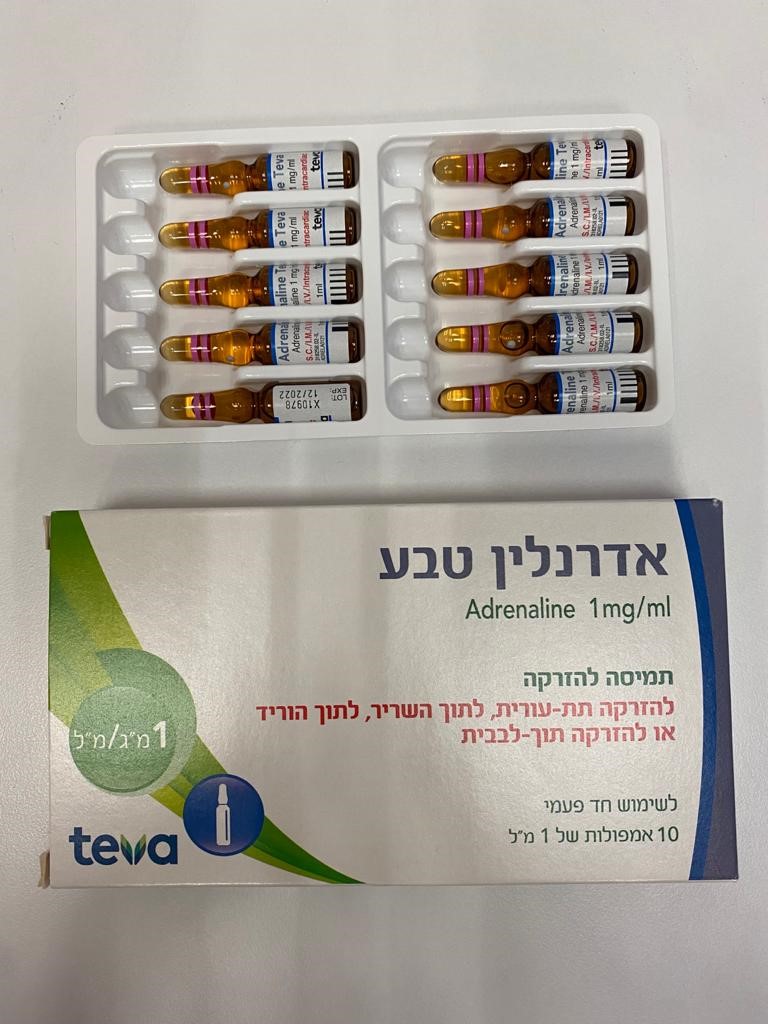
אדרנלין טבע ADRENALINE TEVA (EPINEPHRINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי, תת-עורי, תוך לבבי : I.M, I.V, S.C, INTRACARDIAC
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Adrenaline should be administered with special caution in patients with cerebrovascular insufficiency and in patients with heart disease such as angina pectoris or myocardial infarction, in patients with chronic pulmonary disease and in patients with urinary difficulty due to prostatic hypertrophy. The hypokalaemic effect of adrenaline may be potentiated by other drugs that cause potassium loss, such as corticosteroids, diuretics, aminophylline or theophylline, and periodic checks are therefore advisable. Hypokalaemia may increase susceptibility to cardiac arrhythmias caused by digoxin and other cardiac glycosides (see section 4.5 Interaction with other medicinal products and other forms of interaction). In diabetic patients, doses should be monitored and special caution exercised due to the possible adverse reactions that may occur, particularly in relation to metabolic changes. Special caution is recommended in elderly patients who are more prone to the adverse effects of this medicinal product. Repeated local injections can cause necrosis at the injection site due to vascular vasoconstriction. Injection sites should be alternated. This medicine should not be injected intramuscularly into the buttocks, as adrenaline-induced vasoconstriction reduces the oxygen tension of tissues, enabling anaerobic Clostridium welchii which may be present on the buttocks to multiply and lead to gas gangrene. Due to its vasoconstrictive properties, adrenaline should not be administered into peripheral areas of the body, such as fingers and toes, ear lobe, nose or penis. Patients who frequently receive adrenaline (and other sympathomimetics), such as asthmatic patients, may develop tolerance and therefore require increased doses to achieve the same therapeutic effect. In advanced cases, this may lead to resistance or refractoriness to the clinical effects of this medicinal product. Changes in laboratory test results It should be borne in mind that epinephrine (adrenaline) can alter the values of the following blood test results: increased glucose, falsely increased bilirubin values, increased cholesterol, increased lactic acid (in the form of lactate) and uric acid (urate) – possibly by renal efferent vasoconstriction – and reduced insulin. Although increases in the lactic acid concentration are generally small, adrenaline overdose may be associated with lactic acidosis. Furthermore, as approximately 40% of adrenaline is metabolised to vanillylmandelic acid, urinary vanillylmandelic acid excretion increases if adrenaline is administered. After adrenaline administration, the determination of catecholamines in urine will also be altered. Use in sportspeople Adrenaline is a substance which may give a positive result in anti-doping tests and its use is considered prohibited in competition. However, the use of adrenaline is permitted when administered in association with local anaesthetics or in preparations for local use, for example for nasal or ophthalmological administration. Important information about some of the ingredients of Adrenaline Teva This medicine may rarely cause severe hypersensitivity reactions and bronchospasm because it contains sodium metabisulfite. This medicine contains less than 23 mg sodium (1 mmol) per ampoule; that is to say essentially "sodium-free".
Effects on Driving
4.7 Effects on ability to drive and use machines Not relevant.

שימוש לפי פנקס קופ''ח כללית 1994
Status asthmaticus (in children only), anaphylactic shock, anaphylactoid reaction, cardiac arrest
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
רישום
027 62 21638 21
מחיר
0 ₪
מידע נוסף
