Quest for the right Drug
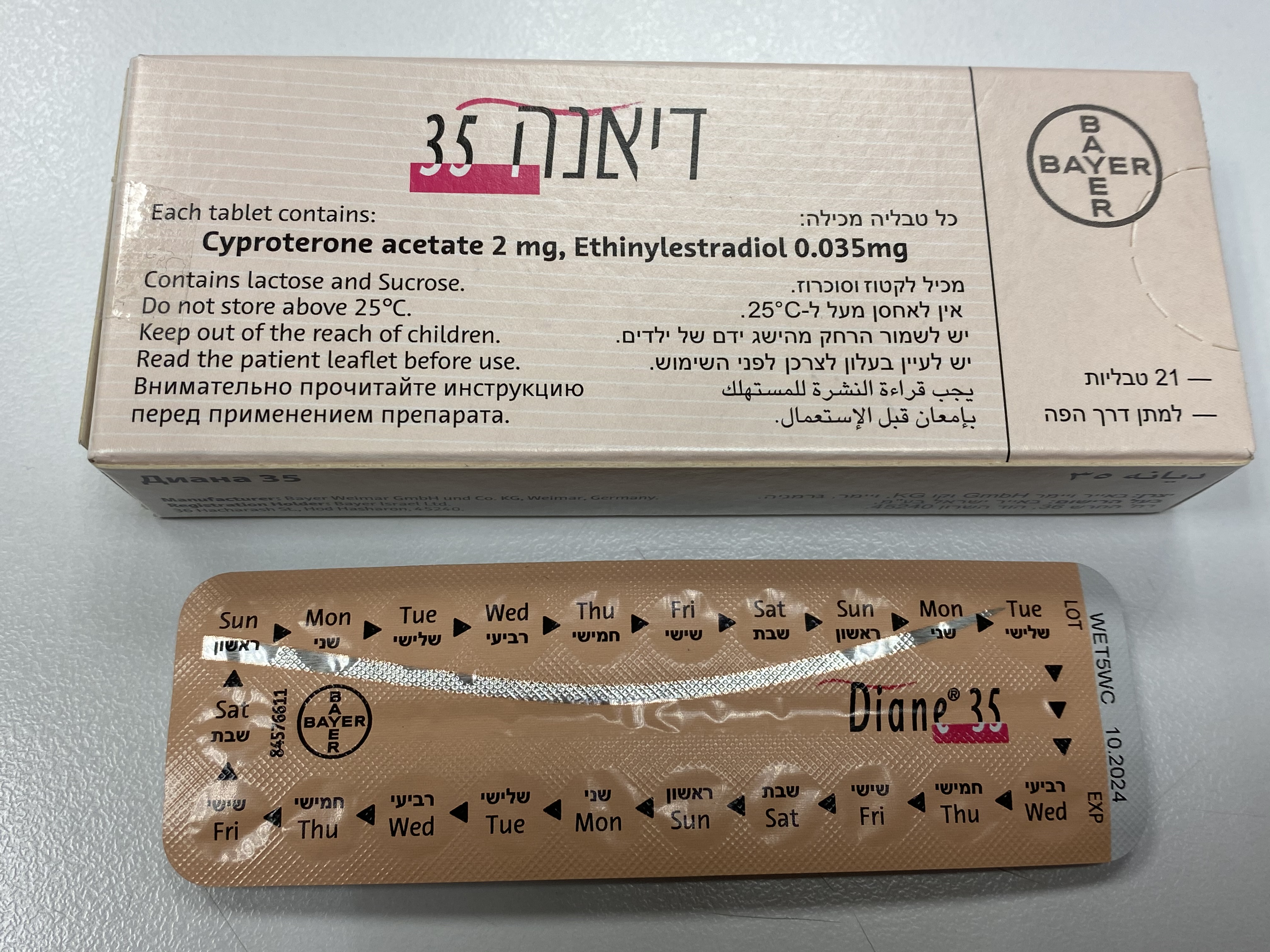
דיאנה 35 DIANE ® 35 (CYPROTERONE ACETATE, ETHINYLESTRADIOL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Sex hormones and modulators of the genital system, antiandrogens and oestrogens. ATC code: G03HB01 Diane 35 blocks androgen-receptors. It also reduces androgen synthesis both by negative feedback effect on the hypothalamo-pituitiary-ovarian systems and by the inhibition of androgen-synthesising enzymes. Although Diane 35 also acts as an oral contraceptive, it is not recommended in women solely for contraception, but should be reserved for those women requiring treatment for the androgen-dependent skin conditions described. Meningioma Based on results from a French epidemiological cohort study, a cumulative dose-dependent association between cyproterone acetate and meningioma has been observed. This study was based on data from the French Health Insurance (CNAM) and included a population of 253,777 women using 50 - 100 mg cyproterone tablets. The incidence of meningioma treated with surgery or radiotherapy was compared between women exposed to high-dose cyproterone acetate (cumulative dose ≥3 g) and women who were slightly exposed to cyproterone acetate (cumulative dose <3 g). A cumulative dose-response relationship was demonstrated. Cumulative dose of Incidence rate HRadj (95% CI)a cyproterone acetate (in patient-years) Slightly exposed (<3 g) 4.5/100,000 Ref. Exposed to ≥3 g 23.8/100,000 6.6 [4.0-11.1] 12 to 36 g 26/100,000 6.4 [3.6-11.5] 36 to 60 g 54.4/100,000 11.3 [5.8-22.2] more than 60 g 129.1/100,000 21.7 [10.8-43.5] a Adjusted based on age as a time-dependent variable and oestrogen at inclusion A cumulative dose of 12g for example can correspond with one year of treatment with 50 mg/day for 20 days each month.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Cyproterone acetate: Following oral administration cyproterone acetate is completely absorbed in a wide dose range. The ingestion of Diane 35 effects a maximum serum level of 15ng cyproterone acetate/ml at 1.6 hours. Thereafter drug serum levels decrease in two disposition phases characterised by half-lives of 0.8 hours and 2.3 days. The total clearance of cyproterone acetate from serum was determined to be 3.6 ml/min/kg. Cyproterone acetate is metabolised by various pathways including hydroxylations and conjugations. The main metabolite in human plasma is the 15β-hydroxy derivative. Some dose parts are excreted unchanged with the bile fluid. Most of the dose is excreted in form of metabolites at a urinary to biliary ratio of 3:7. The renal and biliary excretion was determined to proceed with half-life of 1.9 days. Metabolites from plasma were eliminated at a similar rate (half-life of 1.7 days). Cyproterone acetate is almost exclusively bound to plasma albumin. About 3.5 - 4.0% of total drug levels are present unbound. Because protein binding is non-specific changes in sex hormone binding globulin (SHBG) levels do not affect cyproterone acetate pharmacokinetics. According to the long half-life of the terminal disposition phase from plasma (serum) and the daily intake cyproterone acetate accumulates during one treatment cycle. Mean maximum drug serum levels increased from 15ng/ml (day 1) to 21ng/ml and 24ng/ml at the end of the treatment cycles 1 and 3 respectively. The area under the concentration versus time profile increased 2.2 fold (end of cycle 1) and 2.4 fold (end of cycle 3). Steady state conditions were reached after about 16 days. During long term treatment cyproterone acetate accumulates over treatment cycles by a factor of 2. The absolute bioavailability of cyproterone acetate is almost complete (88% of dose). The relative bioavailability of cyproterone acetate from Diane 35 was 109% when compared to an aqueous microcrystalline suspension. Ethinylestradiol: Orally administered ethinylestradiol is rapidly and completely absorbed. Following ingestion of Diane 35 maximum drug serum levels of about 80pg/ml are reached at 1.7 hours. Thereafter ethinylestradiol plasma levels decrease in two phases characterised by half-lives of 1 - 2 hours and about 20 hours. For analytical reasons these parameters can only be calculated for higher dosages. For ethinylestradiol an apparent volume of distribution of about 5 l/kg and a metabolic clearance rate from plasma of about 5 ml/min/kg were determined. Ethinylestradiol is highly but non-specifically bound to serum albumin. 2% of the drug levels are present unbound. During absorption and first liver passage ethinylestradiol is metabolised resulting in a reduced absolute and variable oral bioavailability. Unchanged drug is not excreted. Ethinylestradiol metabolites are excreted at a urinary to biliary ratio of 4:6 with a half-life of about 1 day. According to the half-life of the terminal disposition phase from plasma and the daily ingestion steady state plasma levels are reached after 3 - 4 days and are higher by 30 - 40% as compared to a single dose. The relative bioavailability (reference: aqueous microcrystalline suspension) of ethinylestradiol was almost complete. The systemic bioavailability of ethinylestradiol might be influenced in both directions by other drugs. There is, however, no interaction with high doses of vitamin C. Ethinylestradiol induces the hepatic synthesis of SHBG and corticosteroid binding globulin (CBG) during continuous use. The extent of SHBG induction, however, is dependent upon the chemical structure and dose of the co-administered progestin. During treatment with Diane 35 SHBG concentrations in serum increased from about 100nmol/l to 300nmol/l and the serum concentrations of CBG were increased from about 50 μg/ml to 95 μg/ml. In vitro, ethinylestradiol is a reversible inhibitor of CYP2C19, CYP1A1 and CYP1A2 as well as a mechanism based inhibitor of CYP3A4/5, CYP2C8 and CYP2J2.

שימוש לפי פנקס קופ''ח כללית 1994
Signs of androgenization in women
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
