Quest for the right Drug
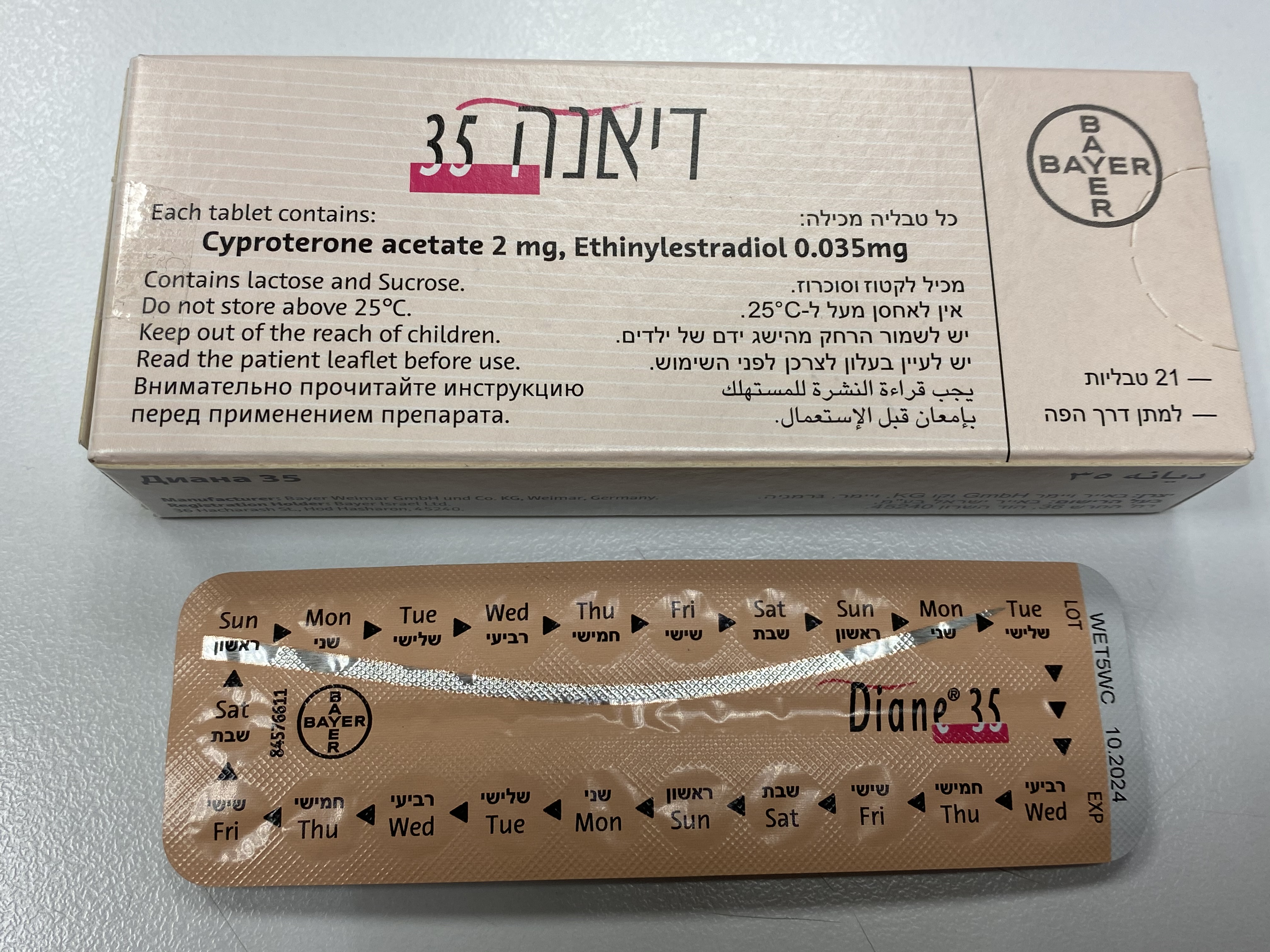
דיאנה 35 DIANE ® 35 (CYPROTERONE ACETATE, ETHINYLESTRADIOL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Method of Administration Oral use Dosage regimen Diane 35 inhibits ovulation and thereby prevents conception. Patients who are using Diane 35 should not therefore use an additional hormonal contraceptive, as this will expose the patient to an excessive dose of hormones and is not necessary for effective contraception. First treatment course: One tablet daily for 21 days, starting on the first day of the menstrual cycle (the first day of menstruation counting as Day 1). Subsequent courses: Each subsequent course is started after 7 tablet-free days have followed the preceding course. When the contraceptive action of Diane 35 is also to be employed, it is essential that the above instructions be rigidly adhered to. Should bleeding fail to occur during the tablet-free interval, the possibility of pregnancy must be excluded before the next pack is started. When changing from an oral contraceptive and relying on the contraceptive action of Diane 35, follow the instructions given below: Changing from 21-day combined oral contraceptives: The first tablet of Diane 35 should be taken on the first day immediately after the end of the previous oral contraceptive course. Additional contraceptive precautions are not required. Changing from a combined Every Day pill (28 day tablets): Diane 35 should be started after taking the last hormone containing tablet from the Every Day Pill pack. The first Diane 35 tablet is taken the next day. Additional contraceptive precautions are not then required. Changing from a progestogen-only pill (POP): The first tablet of Diane 35 should be taken on the first day of bleeding, even if a POP has already been taken on that day. Additional contraceptive precautions are not then required. The remaining progestogen-only pills should be discarded. Post-partum and post-abortum use: After pregnancy, Diane 35 can be started 21 days after a vaginal delivery, provided that the patient is fully ambulant and there are no puerperal complications. Additional contraceptive precautions will be required for the first 7 days of pill taking. Since the first post-partum ovulation may precede the first bleeding, another method of contraception should be used in the interval between childbirth and the first course of tablets. Lactation is contra-indicated with Diane 35. After a first-trimester abortion, Diane 35 may be started immediately in which case no additional contraceptive precautions are required. Duration of Use Time to relief of symptoms is at least three months. The need to continue treatment should be evaluated periodically by the treating physician. Special circumstances requiring additional contraception Incorrect administration: A single delayed tablet should be taken as soon as possible, and if this can be done within 12 hours of the correct time, contraceptive protection is maintained. With longer delays, additional contraception is needed. Only the most recently delayed tablet should be taken, earlier missed tablets being omitted, and additional non-hormonal methods of contraception (except the rhythm or temperature methods) should be used for the next 7 days, while the next 7 tablets are being taken. Additionally, therefore, if tablet(s) have been missed during the last 7 days of a pack, there should be no break before the next pack is started. In this situation, a withdrawal bleed should not be expected until the end of the second pack. Some breakthrough bleeding may occur on tablet taking days but this is not clinically significant. If the patient does not have a withdrawal bleed during the tablet-free interval following the end of the second pack, the possibility of pregnancy must be ruled out before starting the next pack. Gastro-intestinal upset: Vomiting or diarrhoea may reduce the efficacy of oral contraceptives by preventing full absorption. Tablet-taking from the current pack should be continued. Additional non- hormonal methods of contraception (except the rhythm or temperature methods) should be used during the gastro-intestinal upset and for 7 days following the upset. If these 7 days overrun the end of a pack, the next pack should be started without a break. In this situation, a withdrawal bleed should not be expected until the end of the second pack. If the patient does not have a withdrawal bleed during the tablet-free interval following the end of the second pack, the possibility of pregnancy must be ruled out before starting the next pack. Other methods of contraception should be considered if the gastro- intestinal disorder is likely to be prolonged. Additional information on special populations Children and adolescents Diane 35 is only indicated after menarche. Geriatric patients Not applicable. Diane 35 is not indicated after menopause. Patients with hepatic impairment Diane 35 is contraindicated in women with severe hepatic disease as long as liver function values have not returned to normal. See also section 4.3. Patients with renal impairment Diane 35 has not been specifically studied in renally impaired patients. Available data do not suggest a change in treatment in this patient population.

שימוש לפי פנקס קופ''ח כללית 1994
Signs of androgenization in women
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
