Quest for the right Drug
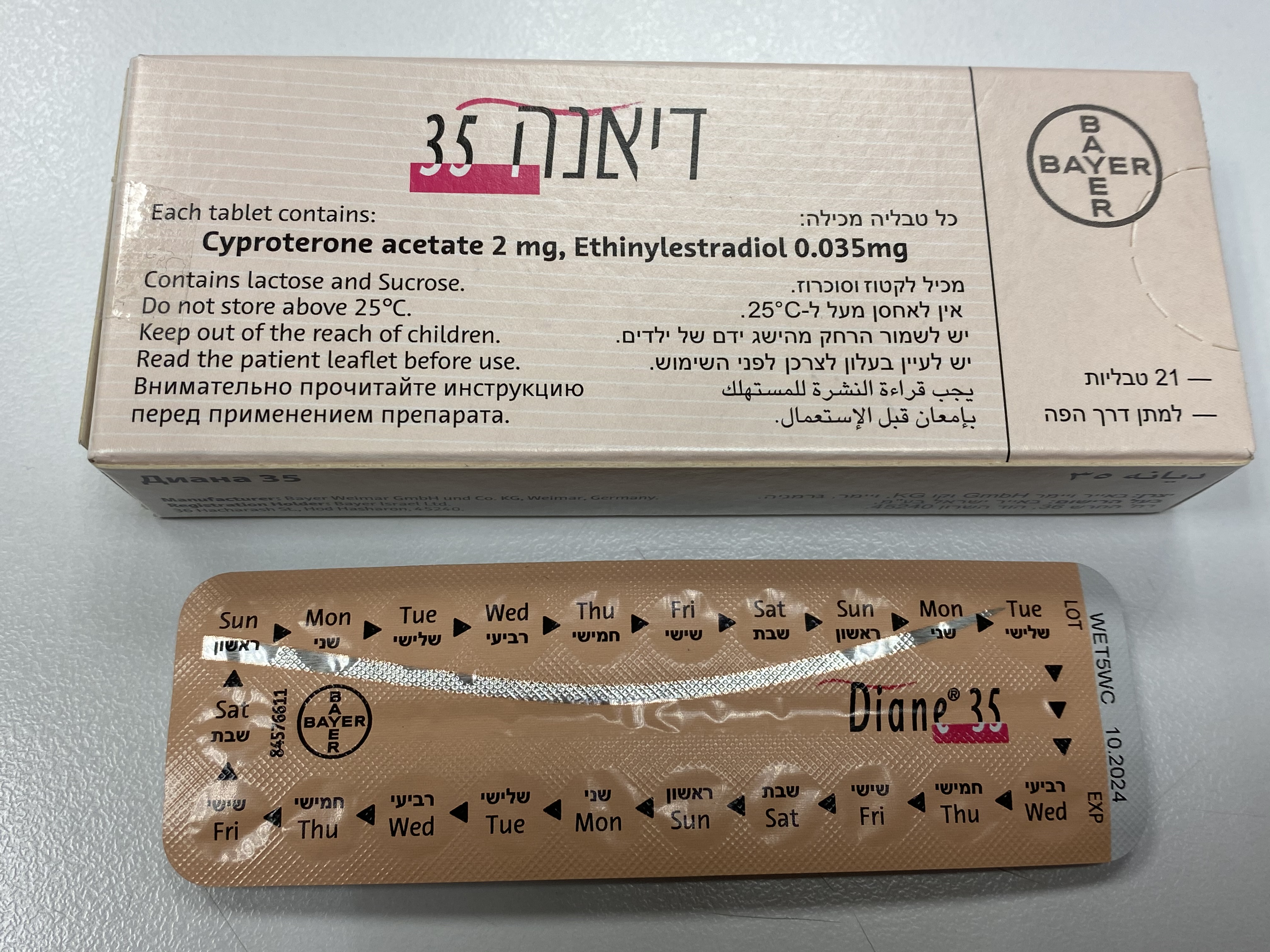
דיאנה 35 DIANE ® 35 (CYPROTERONE ACETATE, ETHINYLESTRADIOL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pregnancy & Lactation : הריון/הנקה
4.6 Pregnancy and lactation Diane 35 is not indicated during pregnancy. If pregnancy occurs during treatment with Diane 35, further intake must be stopped. Animal studies have revealed that feminisation of male foetuses may occur if cyproterone acetate is administered during the phase of embryogenesis at which differentiation of the external genitalia occurs. Although the results of these tests are not necessarily relevant to man, the possibility must be considered that administration of Diane 35 to women after the 45th day of pregnancy could cause feminisation of male foetuses. It follows from this that pregnancy is an absolute contraindication for treatment with Diane 35, and must be excluded before such treatment is begun. The use of Diane 35 during lactation may lead to a reduction in the volume of milk produced and to a change in its composition. Minute amounts of the active substances are excreted with the milk. These amounts may affect the child particularly in the first 6 weeks post-partum. Mothers who are breast- feeding should be advised not to take Diane 35 until the nursing mother has weaned her child off breast milk.

שימוש לפי פנקס קופ''ח כללית 1994
Signs of androgenization in women
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
