Quest for the right Drug
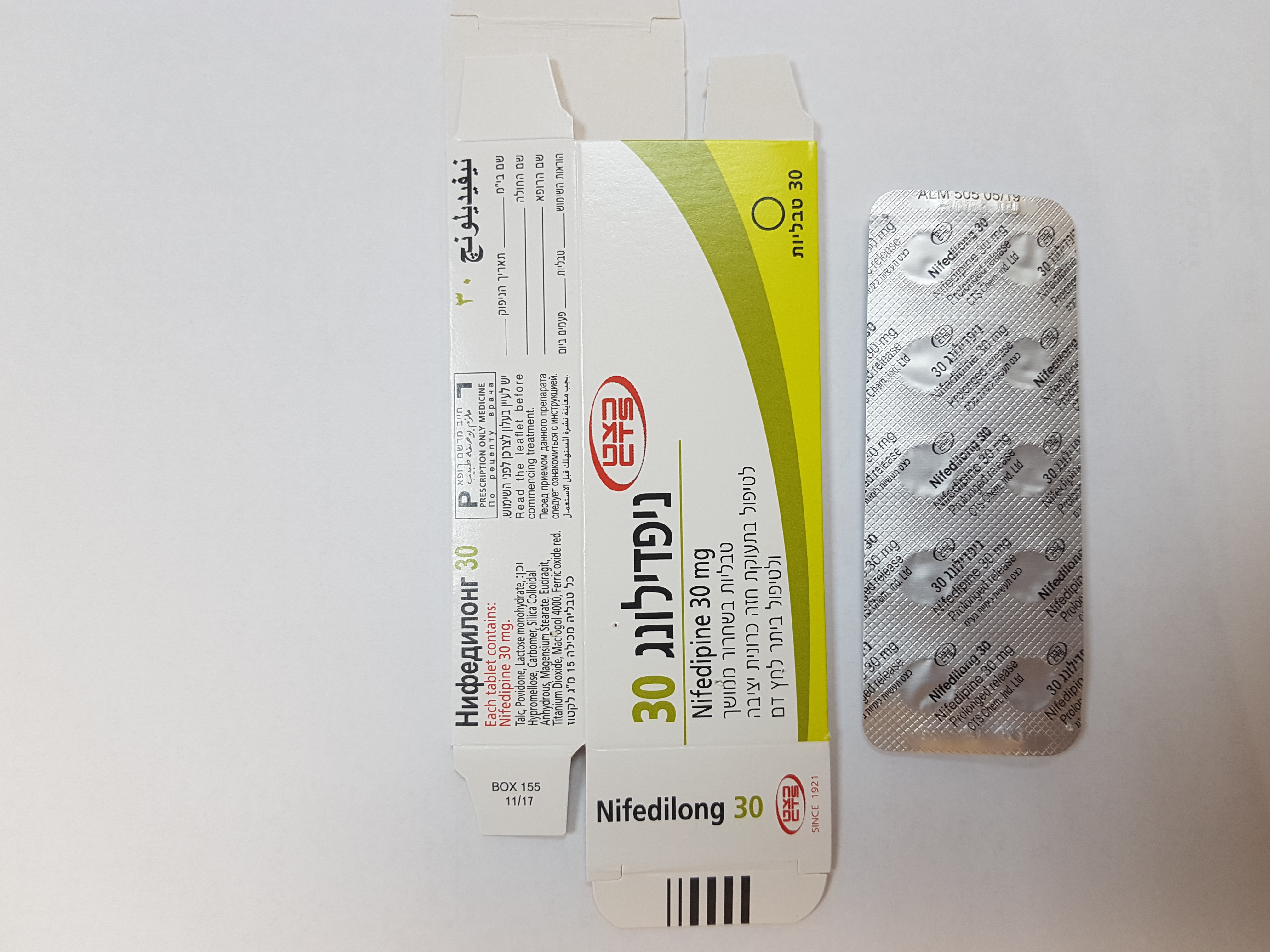
ניפדילונג 30 NIFEDILONG 30 (NIFEDIPINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות בשחרור ממושך : TABLETS PROLONGED RELEASE
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Interactions : אינטראקציות
4.5 Interactions with other medicinal products and other forms of interaction Drugs that affect nifedipine Nifedipine is metabolised via the cytochrome P450 3A4 system, located both in the intestinal mucosa and in the liver. Drugs that are known to either inhibit or to induce this enzyme system may therefore alter the first pass (after oral administration) or the clearance of nifedipine. The extent as well as the duration of interactions should be taken into account when administering nifedipine together with the following drugs: Rifampicin: Rifampicin strongly induces the cytochrome P450 3A4 system. Upon co- administration with rifampicin, the bioavailability of nifedipine is distinctly reduced and thus its efficacy weakened. The use of nifedipine in combination with rifampicin is therefore contraindicated (see Section 4.3). Upon co-administration of known inhibitors of the cytochrome P450 3A4 system, the blood pressure should be monitored and, if necessary, a reduction in the nifedipine dose considered (see Sections 4.2 and 4.4). In the majority of these cases, no formal studies to assess the potential for a drug interaction between nifedipine and the drug(s) listed have been undertaken, thus far. Drugs increasing nifedipine exposure: - macrolide antibiotics (e.g. erythromycin) - anti-HIV protease inhibitors (e.g. ritonavir) - azole anti-mycotics (e.g., ketoconazole) - fluoxetine - nefazodone - quinupristin/dalfopristin - cisapride - valproic acid - cimetidine - diltiazem Upon co-administration of inducers of the cytochrome P450 3A4 system, the clinical response to nifedipine should be monitored and, if necessary, an increase in the nifedipine dose considered. If the dose of nifedipine is increased during co- administration of both drugs, a reduction of the nifedipine dose should be considered when the treatment is discontinued. Drugs decreasing nifedipine exposure: - rifampicin (see above) - phenytoin - carbamazepine - phenobarbital Effects of nifedipine on other drugs Nifedipine may increase the blood pressure lowering effect of concomitant applied antihypertensives. When nifedipine is administered simultaneously with ß-receptor blockers the patient should be carefully monitored, since deterioration of heart failure is also known to develop in isolated cases. Digoxin: The simultaneous administration of nifedipine and digoxin may lead to reduced digoxin clearance and, hence, an increase in the plasma digoxin level. The patient should therefore be subjected to precautionary checks for symptoms of digoxin overdosage and, if necessary, the glycoside dose should be reduced. Quinidine: Co-administration of nifedipine with quinidine may lower plasma quinidine levels, and after discontinuation of nifedipine, a distinct increase in plasma quinidine levels may be observed in individual cases. Consequently, when nifedipine is either additionally administered or discontinued, monitoring of the quinidine plasma concentration, and if necessary, adjustment of the quinidine dose is recommended. Blood pressure should be carefully monitored and, if necessary, the dose of nifedipine should be decreased. Tacrolimus: Tacrolimus is metabolised via the cytochrome P450 3A4 system. Published data indicate that the dose of tacrolimus administered simultaneously with nifedipine may be reduced in individual cases. Upon co-administration of both drugs, the tacrolimus plasma concentrations should be monitored and, if necessary, a reduction in the tacrolimus dose considered. Drug food interactions Grapefruit juice inhibits the cytochrome P450 3A4 system. Administration of nifedipine together with grapefruit juice thus results in elevated plasma concentrations and prolonged action of nifedipine due to a decreased first pass metabolism or reduced clearance. As a consequence, the blood pressure lowering effect of nifedipine may be increased. After regular intake of grapefruit juice, this effect may last for at least three days after the last ingestion of grapefruit juice. Ingestion of grapefruit/grapefruit juice is therefore to be avoided while taking nifedipine (see Section 4.2). Other forms of interaction Nifedipine may increase the spectrophotometric values of urinary vanillylmandelic acid, falsely. However, HPLC measurements are unaffected.

שימוש לפי פנקס קופ''ח כללית 1994
Hypertension, vasospastic angina (Prinzmetal), chronic stable angina
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
