Quest for the right Drug
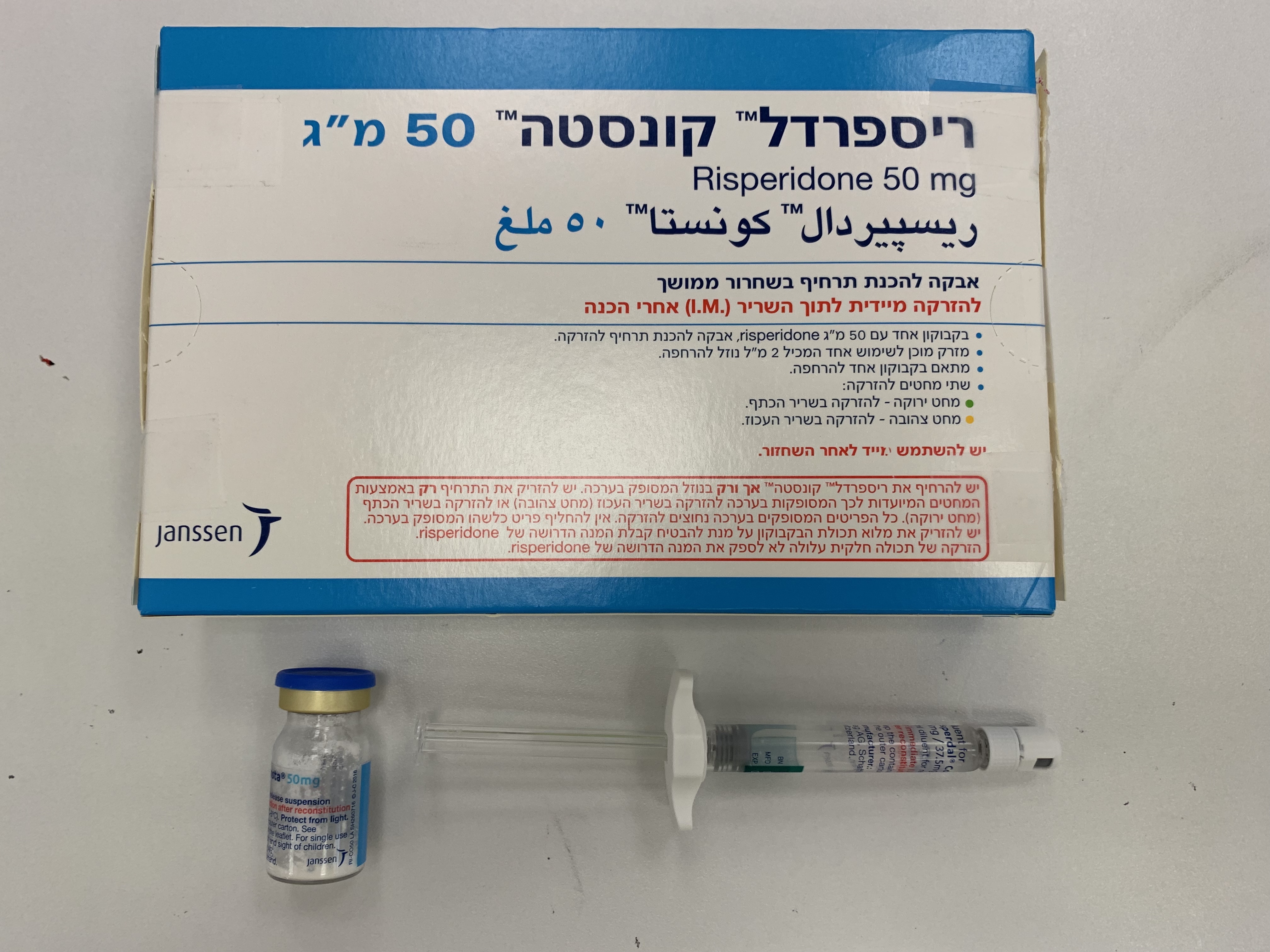
ריספרדל קונסטה 25 מ"ג RISPERDAL CONSTA 25 MG (RISPERIDONE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
אבקה לתרחיף להזרקה : POWDER FOR SUSPENSION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
5 WARNINGS AND PRECAUTIONS 5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. RISPERDAL CONSTA®(risperidone) is not approved for the treatment of dementia-related psychosis [see Boxed Warning]. 5.2 Cerebrovascular Adverse Events, Including Stroke, in Elderly Patients with Dementia-Related Psychosis Cerebrovascular adverse events (e.g., stroke, transient ischemic attack), including fatalities, were reported in patients (mean age 85 years; range 73-97) in trials of oral risperidone in elderly patients with dementia-related psychosis. In placebo-controlled trials, there was a significantly higher incidence of cerebrovascular adverse events in patients treated with oral risperidone compared to patients treated with placebo. RISPERDAL CONSTA® is not approved for the treatment of patients with dementia-related psychosis. [See also Boxed Warning and Warnings and Precautions (5.1)] 5.3 Neuroleptic Malignant Syndrome Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex, has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status including delirium, and autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. If NMS is suspected, immediately discontinue RISPERDAL CONSTA® and provide symptomatic treatment and monitoring. 5.4 Tardive Dyskinesia Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown. The risk of developing tardive dyskinesia and the likelihood that it will become irreversible increase with the duration of treatment and the cumulative dose. The syndrome can develop after relatively brief treatment periods, even at low doses. It may also occur after discontinuation of treatment. Tardive dyskinesia may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown. Given these considerations, RISPERDAL CONSTA® should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients: (1) who suffer from a chronic illness that is known to respond to antipsychotic drugs, and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the lowest dose and the shortest duration of treatment producing a satisfactory clinical response. Periodically reassess the need for continued treatment. If signs and symptoms of tardive dyskinesia appear in a patient treated with RISPERDAL CONSTA®, drug discontinuation should be considered. However, some patients may require treatment with RISPERDAL CONSTA® despite the presence of the syndrome. 5.5 Metabolic Changes Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile. Hyperglycemia and Diabetes Mellitus Hyperglycemia and diabetes mellitus, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, have been reported in patients treated with atypical antipsychotics including RISPERDAL®. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of treatment-emergent hyperglycemia-related adverse events in patients treated with the atypical antipsychotics. Precise risk estimates for hyperglycemia-related adverse events in patients treated with atypical antipsychotics are not available. Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics, including RISPERDAL® should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics, including RISPERDAL® should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics, including RISPERDAL should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics, including RISPERDAL®, should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic, including RISPERDAL®, was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of RISPERDAL®. Pooled data from 3 double-blind, placebo-controlled studies in subjects with schizophrenia and 4 double-blind, placebo-controlled monotherapy studies in subjects with bipolar mania with oral risperidone are presented in Table 1. Table 1. Change in Random Glucose From Seven Placebo-Controlled, 3- to 8-Week, Fixed- or Flexible-Dose Studies in Adult Subjects With Schizophrenia or Bipolar Mania With Oral Risperidone RISPERDAL® Placebo 1-8 mg/day >8-16 mg/day Mean change from baseline (mg/dL) N=555 N=748 N=164 Serum Glucose -1.4 0.8 0.6 Proportion of patients with shifts Serum Glucose 0.6% 0.4% 0% (<140 mg/dL to ≥200 mg/dL) (3/525) (3/702) (0/158) In longer-term, controlled and uncontrolled studies in adult subjects, RISPERDAL® was associated with a mean change in glucose of +2.8 mg/dL at Week 24 (N=151) and +4.1 mg/dL at Week 48 (N=50). Dyslipidemia Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics. Pooled data from 7 placebo-controlled, 3- to 8- week, fixed- or flexible-dose studies in adult subjects with schizophrenia or bipolar mania are presented in Table 2. Table 2. Change in Random Lipids From Seven Placebo-Controlled, 3- to 8-Week, Fixed- or Flexible-Dose Studies in Adult Subjects With Schizophrenia or Bipolar Mania With Oral Risperidone RISPERDAL® Placebo 1-8 mg/day >8-16 mg/day Mean change from baseline (mg/dL) Cholesterol N=559 N=742 N=156 Change from baseline 0.6 6.9 1.8 Triglycerides N=183 N=307 N=123 Change from baseline -17.4 -4.9 -8.3 Proportion of patients With Shifts Cholesterol 2.7% 4.3% 6.3% (<200 mg/dL to ≥240 mg/dL) (10/368) (22/516) (6/96) Triglycerides 1.1% 2.7% 2.5% (<500 mg/dL to ≥500 mg/dL) (2/180) (8/301) (3/121) In longer-term, controlled and uncontrolled studies, RISPERDAL® was associated with a mean change in (a) non-fasting cholesterol of +4.4 mg/dL at Week 24 (N=231) and +5.5 mg/dL at Week 48 (N=86); and (b) non-fasting triglycerides of +19.9 mg/dL at Week 24 (N=52). Weight Gain Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended. Data from a placebo-controlled, 12-week, fixed-dose study in adult subjects with schizophrenia are presented in Table 3. Table 3. Mean Change in Body Weight (kg) and the Proportion of Subjects With ≥7% Gain in Body Weight From a Placebo-Controlled, 12-Week, Fixed-Dose Study in Adult Subjects With Schizophrenia RISPERDAL CONSTA® Placebo 25 mg 50 mg (N=83) (N=90) (N=87) Weight (kg) Change from baseline -1.4 0.5 1.2 Weight Gain ≥7% increase from baseline 6% 10% 8% In an uncontrolled, longer-term, open-label study, RISPERDAL CONSTA® was associated with a mean change in weight of +2.1 kg at Week 24 (N=268) and +2.8 kg at Week 50 (N=199). 5.6 Hyperprolactinemia As with other drugs that antagonize dopamine D2 receptors, risperidone elevates prolactin levels and the elevation persists during chronic administration. Risperidone is associated with higher levels of prolactin elevation than other antipsychotic agents. Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with previously detected breast cancer. An increase in pituitary gland, mammary gland, and pancreatic islet cell neoplasia (mammary adenocarcinomas, pituitary and pancreatic adenomas) was observed in the risperidone carcinogenicity studies conducted in mice and rats [see Nonclinical Toxicology (13.1)]. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans; the available evidence is considered too limited to be conclusive at this time. 5.7 Orthostatic Hypotension RISPERDAL CONSTA® may induce orthostatic hypotension associated with dizziness, tachycardia, and in some patients, syncope, especially during the initial dose-titration period with oral risperidone, probably reflecting its alpha-adrenergic antagonistic properties. Syncope was reported in 0.8% (12/1499 patients) of patients treated with RISPERDAL CONSTA® in multiple- dose studies. Patients should be instructed in nonpharmacologic interventions that help to reduce the occurrence of orthostatic hypotension (e.g., sitting on the edge of the bed for several minutes before attempting to stand in the morning and slowly rising from a seated position). RISPERDAL CONSTA® should be used with particular caution in (1) patients with known cardiovascular disease (history of myocardial infarction or ischemia, heart failure, or conduction abnormalities), cerebrovascular disease, and conditions which would predispose patients to hypotension, e.g., dehydration and hypovolemia, and (2) in the elderly and patients with renal or hepatic impairment. Monitoring of orthostatic vital signs should be considered in all such patients, and a dose reduction should be considered if hypotension occurs. Clinically significant hypotension has been observed with concomitant use of oral RISPERDAL® and antihypertensive medication. 5.8 Falls Somnolence, postural hypotension, motor and sensory instability have been reported with the use of antipsychotics, including RISPERDAL CONSTA®, which may lead to falls and, consequently, fractures or other fall-related injuries. For patients, particularly the elderly, with diseases, conditions, or medications that could exacerbate these effects, assess the risk of falls when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy. 5.9 Leukopenia, Neutropenia, and Agranulocytosis Class Effect: In clinical trial and/or postmarketing experience, events of leukopenia/neutropenia have been reported temporally related to antipsychotic agents, including RISPERDAL CONSTA®. Agranulocytosis has also been reported. Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) and a history of drug-induced leukopenia/neutropenia. Patients with a history of a clinically significant low WBC or a drug-induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and discontinuation of RISPERDAL CONSTA® should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors. Patients with clinically significant neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue RISPERDAL CONSTA® and have their WBC followed until recovery. 5.10 Potential for Cognitive and Motor Impairment Somnolence was reported by 5% of patients treated with RISPERDAL CONSTA® in multiple-dose trials. Since risperidone has the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that treatment with RISPERDAL CONSTA® does not affect them adversely. 5.11 Seizures During premarketing testing, seizures occurred in 0.3% (5/1499 patients) of patients treated with RISPERDAL CONSTA®. Therefore, RISPERDAL CONSTA® should be used cautiously in patients with a history of seizures. 5.12 Dysphagia Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in patients with advanced Alzheimer’s dementia. RISPERDAL CONSTA® and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia. [see also Boxed Warning and Warnings and Precautions (5.1)] 5.13 Priapism Priapism has been reported during postmarketing surveillance [see Adverse Reactions6.2]. Severe priapism may require surgical intervention. 5.14 Body Temperature Regulation Disruption of body temperature regulation has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with oral RISPERDAL® or RISPERDAL CONSTA® use. Caution is advised when prescribing RISPERDAL CONSTA® for patients who will be exposed to temperature extremes. 5.15 Administration RISPERDAL CONSTA® should be injected into the deltoid or gluteal muscle, and care must be taken to avoid inadvertent injection into a blood vessel. [see Dosage and Administration (2) and Adverse Reactions (6.7)] 5.16 Osteodystrophy and Tumors in Animals RISPERDAL CONSTA® produced osteodystrophy in male and female rats in a 1-year toxicity study and a 2-year carcinogenicity study at a dose of 40 mg/kg administered IM every 2 weeks. RISPERDAL CONSTA® produced renal tubular tumors (adenoma, adenocarcinoma) and adrenomedullary pheochromocytomas in male rats in the 2-year carcinogenicity study at 40 mg/kg administered IM every 2 weeks. In addition, RISPERDAL CONSTA® produced an increase in a marker of cellular proliferation in renal tissue in males in the 1-year toxicity study and in renal tumor-bearing males in the 2-year carcinogenicity study at 40 mg/kg administered IM every 2 weeks. (Cellular proliferation was not measured at the low dose or in females in either study.) The effect dose for osteodystrophy and the tumor findings is 8 times the IM maximum recommended human dose (MRHD) (50 mg) on a mg/m2 basis and is associated with a plasma exposure (AUC) 2 times the expected plasma exposure (AUC) at the IM MRHD. The no-effect dose for these findings was 5 mg/kg (equal to the IM MRHD on a mg/m2 basis). Plasma exposure (AUC) at the no-effect dose was one third the expected plasma exposure (AUC) at the IM MRHD. Neither the renal or adrenal tumors, nor osteodystrophy, were seen in studies of orally administered risperidone. Osteodystrophy was not observed in dogs at doses up to 14 times (based on AUC) the IM MRHD in a 1-year toxicity study. The renal tubular and adrenomedullary tumors in male rats and other tumor findings are described in more detail in Section 13.1 (Carcinogenicity, Mutagenesis, Impairment of Fertility). The relevance of these findings to human risk is unknown. 6 ADVERSE REACTIONS The following are discussed in more detail in other sections of the labeling: • Increased mortality in elderly patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions (5.1)] • Cerebrovascular adverse events, including stroke, in elderly patients with dementia-related psychosis [see Warnings and Precautions (5.2)] • Neuroleptic malignant syndrome [see Warnings and Precautions (5.3)] • Tardive dyskinesia [see Warnings and Precautions (5.4)] • Metabolic changes [see Warnings and Precautions (5.5)] • Hyperprolactinemia [see Warnings and Precautions (5.6)] • Orthostatic hypotension [see Warnings and Precautions (5.7)] • Falls [see Warnings and Precautions (5.8)] • Leukopenia/Neutropenia and Agranulocytosis [see Warnings and Precautions (5.9)] • Potential for cognitive and motor impairment [see Warnings and Precautions (5.10)] • Seizures [see Warnings and Precautions (5.11)] • Dysphagia [see Warnings and Precautions (5.12)] • Priapism [see Warnings and Precautions (5.13)] • Disruption of body temperature regulation [see Warnings and Precautions (5.14)] • Avoidance of inadvertent injection into a blood vessel [see Warnings and Precautions (5.15)] • Osteodystrophy and tumors in animals [see Warnings and Precautions (5.16)] The most common adverse reactions in clinical trials in patients with schizophrenia (≥ 5%) were: headache, parkinsonism, dizziness, akathisia, fatigue, constipation, dyspepsia, sedation, weight increased, pain in extremity, and dry mouth. The most common adverse reactions in the double- blind, placebo-controlled periods of the bipolar disorder trials were weight increased (5% in the monotherapy trial) and tremor and parkinsonism (≥ 10% in the adjunctive treatment trial). The most common adverse reactions that were associated with discontinuation from the 12-week double-blind, placebo-controlled trial in patients with schizophrenia (causing discontinuation in ≥1% of patients) were agitation, depression, anxiety, and akathisia. Adverse reactions that were associated with discontinuation from the double-blind, placebo-controlled periods of the bipolar disorder trials were hyperglycemia (one patient in the monotherapy trial) and hypokinesia and tardive dyskinesia (one patient each in the adjunctive treatment trial). The data described in this section are derived from a clinical trial database consisting of 2392 patients exposed to one or more doses of RISPERDAL CONSTA® for the treatment of schizophrenia. Of these 2392 patients, 332 were patients who received RISPERDAL CONSTA® while participating in a 12-week double-blind, placebo-controlled trial. Two hundred two (202) of the 332 were schizophrenia patients who received 25 mg or 50 mg RISPERDAL CONSTA®. The conditions and duration of treatment with RISPERDAL CONSTA® in the other clinical trials varied greatly and included (in overlapping categories) double-blind, fixed- and flexible-dose, placebo- or active-controlled studies and open-label phases of studies, inpatients and outpatients, and short-term (up to 12 weeks) and longer-term (up to 4 years) exposures. Safety was assessed by collecting adverse events and performing physical examinations, vital signs, body weights, laboratory analyses, and ECGs. In addition to the studies in patients with schizophrenia, safety data are presented from a trial assessing the efficacy and safety of RISPERDAL CONSTA® when administered as monotherapy for maintenance treatment in patients with bipolar I disorder. The subjects in this multi-center, double-blind, placebo-controlled study were adult patients who met DSM-IV criteria for Bipolar Disorder Type I and who were stable on risperidone (oral or long-acting injection), were stable on other antipsychotics or mood stabilizers, or were experiencing an acute episode. After a 3-week period of treatment with open-label oral risperidone (N=440), subjects who demonstrated an initial response to oral risperidone in this period and those who were stable on risperidone (oral or long- acting injection) at study entry entered into a 26-week stabilization period of open-label RISPERDAL CONSTA® (N=501). Subjects who demonstrated a maintained response during this period were then randomized into a 24-month double-blind, placebo-controlled period in which they received RISPERDAL® CONSTA® (N=154) or placebo (N=149) as monotherapy. Subjects who relapsed or who completed the double-blind period could choose to enter an 8-week open- label RISPERDAL CONSTA® extension period (N=160). Safety data are also presented from a trial assessing the efficacy and safety of RISPERDAL CONSTA® when administered as adjunctive maintenance treatment in patients with bipolar disorder. The subjects in this multi-center, double-blind, placebo-controlled study were adult patients who met DSM-IV criteria for Bipolar Disorder Type I or Type II and who experienced at least 4 episodes of mood disorder requiring psychiatric/clinical intervention in the previous 12 months, including at least 2 episodes in the 6 months prior to the start of the study. At the start of this study, all patients (N=275) entered into a 16-week open-label treatment phase in which they received RISPERDAL CONSTA® in addition to continuing their treatment as usual, which consisted of various mood stabilizers (primarily lithium and valproate), antidepressants, and/or anxiolytics. Patients who reached remission at the end of this 16-week open-label treatment phase (N=139) were then randomized into a 52-week double-blind, placebo-controlled phase in which they received RISPERDAL CONSTA® (N=72) or placebo (n=67) as adjunctive treatment in addition to continuing their treatment as usual. Patients who did not reach remission at the end of the 16-week open-label treatment phase could choose to continue to receive RISPERDAL CONSTA® as adjunctive therapy in an open-label manner, in addition to continuing their treatment as usual, for up to an additional 36 weeks as clinically indicated for a total period of up to 52 weeks; these patients (N=70) were also included in the evaluation of safety. Adverse events during exposure to study treatment were obtained by general inquiry and recorded by clinical investigators using their own terminology. Consequently, to provide a meaningful estimate of the proportion of individuals experiencing adverse events, events were grouped in standardized categories using MedDRA terminology. Throughout this section, adverse reactions are reported. Adverse reactions are adverse events that were considered to be reasonably associated with the use of RISPERDAL CONSTA® (adverse drug reactions) based on the comprehensive assessment of the available adverse event information. A causal association for RISPERDAL CONSTA® often cannot be reliably established in individual cases. Further, because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. The majority of all adverse reactions were mild to moderate in severity. 6.1 Clinical Trials Experience Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials - Schizophrenia Table 4 lists the adverse reactions reported in 2% or more of RISPERDAL CONSTA®-treated patients with schizophrenia in one 12-week double-blind, placebo-controlled trial. Table 4. Adverse Reactions in ≥2% of RISPERDAL CONSTA®-Treated Patients with Schizophrenia in a 12-Week Double-Blind, Placebo-Controlled Trial Percentage of Patients Reporting Event RISPERDAL CONSTA® Placebo System/Organ Class 25 mg 50 mg Adverse Reaction (N=99) (N=103) (N=98) Eye disorders Vision blurred 2 3 0 Gastrointestinal disorders Constipation 5 7 1 Dry mouth 0 7 1 Dyspepsia 6 6 0 Nausea 3 4 5 Toothache 1 3 0 Salivary hypersecretion 4 1 0 General disorders and administration site conditions Fatigue* 3 9 0 Edema peripheral 2 3 1 Pain 4 1 0 Pyrexia 2 1 0 Infections and infestations Upper respiratory tract infection 2 0 1 Investigations Weight increased 5 4 2 Weight decreased 4 1 1 Musculoskeletal and connective tissue disorders Pain in extremity 6 2 1 Nervous system disorders Headache 15 21 12 Parkinsonism* 8 15 9 Dizziness 7 11 6 Akathisia* 4 11 6 Sedation* 5 6 3 Tremor 0 3 0 Syncope 2 1 0 Hypoesthesia 2 0 0 Respiratory, thoracic and mediastinal disorders Cough 4 2 3 Sinus congestion 2 0 0 Skin and subcutaneous tissue disorders Acne 2 2 0 Dry skin 2 0 0 * Fatigue includes fatigue and asthenia. Parkinsonism includes extrapyramidal disorder, musculoskeletal stiffness, muscle rigidity, and bradykinesia. Akathisia includes akathisia and restlessness. Sedation includes sedation and somnolence. Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials – Bipolar Disorder Table 5 lists the treatment-emergent adverse reactions reported in 2% or more of RISPERDAL CONSTA®-treated patients in the 24-month double-blind, placebo-controlled treatment period of the trial assessing the efficacy and safety of RISPERDAL CONSTA® when administered as monotherapy for maintenance treatment in patients with Bipolar I Disorder. Table 5. Adverse Reactions in ≥2% of Patients with Bipolar I Disorder Treated with RISPERDAL CONSTA® as Monotherapy in a 24-Month Double-Blind, Placebo-Controlled Trial Percentage of Patients Reporting Event System/Organ Class RISPERDALCONSTA® Placebo Adverse Reaction (N=154) (N=149) Investigations Weight increased 5 1 Nervous system disorders Dizziness 3 1 Vascular disorders Hypertension 3 1 Table 6 lists the treatment-emergent adverse reactions reported in 4% or more of patients in the 52-week double-blind, placebo-controlled treatment phase of a trial assessing the efficacy and safety of RISPERDAL CONSTA® when administered as adjunctive maintenance treatment in patients with bipolar disorder. Table 6. Adverse Reactions in ≥ 4% of Patients with Bipolar Disorder Treated with RISPERDAL CONSTA® as Adjunctive Therapy in a 52-Week Double-Blind, Placebo-Controlled Trial Percentage of Patients Reporting Event + Placebo + Treatment Treatment as Usuala System/Organ Class as Usuala Adverse Reaction (N=72) (N=67) General disorders and administration site conditions Gait abnormal 4 0 Infections and infestations Upper respiratory tract infection 6 3 Investigations Weight increased 7 1 Metabolism and nutrition disorders Decreased appetite 6 1 Increased appetite 4 0 Musculoskeletal and connective tissue disorders Arthralgia 4 3 Nervous system disorders Tremor 24 16 Parkinsonismb 15 6 Dyskinesiab 6 3 Sedationc 7 1 Disturbance in attention 4 0 Reproductive system and breast disorders Amenorrhea 4 1 Respiratory, thoracic and mediastinal disorders Cough 4 1 a ® Patients received double-blind RISPERDAL CONSTA or placebo in addition to continuing their treatment as usual, which included mood stabilizers, antidepressants, and/or anxiolytics. b Parkinsonism includes muscle rigidity, hypokinesia, cogwheel rigidity, and bradykinesia. Dyskinesia includes muscle twitching and dyskinesia. c Sedation includes sedation and somnolence. Other Adverse Reactions Observed During the Clinical Trial Evaluation of Risperidone The following additional adverse reactions occurred in < 2% of the RISPERDAL CONSTA®- treated patients in the above schizophrenia double-blind, placebo-controlled trial dataset, in < 2% of the RISPERDAL CONSTA®-treated patients in the above double-blind, placebo-controlled period of the monotherapy bipolar disorder trial dataset, or in < 4% of the RISPERDAL CONSTA®-treated patients in the above double-blind, placebo-controlled period of the adjunctive treatment bipolar disorder trial dataset. The following also includes additional adverse reactions reported at any frequency in RISPERDAL CONSTA®-treated patients who participated in the open-label phases of the above bipolar disorder studies and in other studies, including double- blind, active controlled and open-label studies in schizophrenia and bipolar disorder. Blood and lymphatic system disorders: anemia, neutropenia Cardiac disorders: tachycardia, atrioventricular block first degree, palpitations, sinus bradycardia, bundle branch block left, bradycardia, sinus tachycardia, bundle branch block right Ear and labyrinth disorders: ear pain, vertigo Endocrine disorders: hyperprolactinemia Eye disorders: conjunctivitis, visual acuity reduced Gastrointestinal disorders: diarrhea, vomiting, abdominal pain upper, abdominal pain, stomach discomfort, gastritis General disorders and administration site conditions: injection site pain, chest discomfort, chest pain, influenza like illness, sluggishness, malaise, induration, injection site induration, injection site swelling, injection site reaction, face edema Immune system disorders: hypersensitivity Infections and infestations: nasopharyngitis, influenza, bronchitis, urinary tract infection, rhinitis, respiratory tract infection, ear infection, pneumonia, lower respiratory tract infection, pharyngitis, sinusitis, viral infection, infection, localized infection, cystitis, gastroenteritis, subcutaneous abscess Injury and poisoning: fall, procedural pain Investigations: blood prolactin increased, alanine aminotransferase increased, electrocardiogram abnormal, gamma-glutamyl transferase increased, blood glucose increased, hepatic enzyme increased, aspartate aminotransferase increased, electrocardiogram QT prolonged, glucose urine present Metabolism and nutritional disorders: anorexia, hyperglycemia Musculoskeletal, connective tissue and bone disorders: posture abnormal, myalgia, back pain, buttock pain, muscular weakness, neck pain, musculoskeletal chest pain Nervous system disorders: coordination abnormal, dystonia, tardive dyskinesia, drooling, paresthesia, dizziness postural, convulsion, akinesia, hypokinesia, dysarthria Psychiatric disorders: insomnia, agitation, anxiety, sleep disorder, depression, initial insomnia, libido decreased, nervousness Renal and urinary disorders: urinary incontinence Reproductive system and breast disorders: galactorrhea, oligomenorrhea, erectile dysfunction, sexual dysfunction, ejaculation disorder, gynecomastia, breast discomfort, menstruation irregular, menstruation delayed, menstrual disorder, ejaculation delayed Respiratory, thoracic and mediastinal disorders: nasal congestion, pharyngolaryngeal pain, dyspnea, rhinorrhea Skin and subcutaneous tissue disorders: rash, eczema, pruritus generalized, pruritus Vascular disorders: hypotension, orthostatic hypotension Additional Adverse Reactions Reported with Oral RISPERDAL® The following is a list of additional adverse reactions that have been reported during the clinical trial evaluation of oral RISPERDAL®, regardless of frequency of occurrence: Blood and Lymphatic Disorders: granulocytopenia Cardiac Disorders: atrioventricular block Ear and Labyrinth Disorders: tinnitus Eye Disorders: ocular hyperemia, eye discharge, eye rolling, eyelid edema, eye swelling, eyelid margin crusting, dry eye, lacrimation increased, photophobia, glaucoma Gastrointestinal Disorders: abdominal pain upper, dysphagia, fecaloma, abdominal discomfort, fecal incontinence, lip swelling, cheilitis, aptyalism General Disorders: thirst, feeling abnormal, gait disturbance, pitting edema, edema, chills, discomfort, generalized edema, drug withdrawal syndrome, peripheral coldness Immune System Disorders: drug hypersensitivity Infections and Infestations: tonsillitis, eye infection, cellulitis, otitis media, onychomycosis, acarodermatitis, bronchopneumonia, respiratory tract infection, tracheobronchitis, otitis media chronic Investigations: body temperature increased, heart rate increased, eosinophil count increased, white blood cell count decreased, hemoglobin decreased, blood creatine phosphokinase increased, hematocrit decreased, body temperature decreased, blood pressure decreased, transaminases increased Metabolism and Nutrition Disorders: polydipsia Musculoskeletal, Connective Tissue, and Bone Disorders: joint swelling, joint stiffness, rhabdomyolysis, torticollis Nervous System Disorders: hypertonia, balance disorder, dysarthria, unresponsive to stimuli, depressed level of consciousness, movement disorder, hypokinesia, parkinsonian rest tremor, transient ischemic attack, cerebrovascular accident, masked facies, speech disorder, loss of consciousness, muscle contractions involuntary, akinesia, cerebral ischemia, cerebrovascular disorder, neuroleptic malignant syndrome, diabetic coma, head titubation Psychiatric Disorders: blunted affect, confusional state, middle insomnia, listlessness, anorgasmia Renal and Urinary Disorders: enuresis, dysuria, pollakiuria Reproductive System and Breast Disorders: vaginal discharge, retrograde ejaculation, ejaculation disorder, ejaculation failure, breast enlargement Respiratory, Thoracic, and Mediastinal Disorders: epistaxis, wheezing, pneumonia aspiration, dysphonia, productive cough, pulmonary congestion, respiratory tract congestion, rales, respiratory disorder, hyperventilation, nasal edema Skin and Subcutaneous Tissue Disorders: erythema, skin discoloration, skin lesion, skin disorder, rash erythematous, rash papular, hyperkeratosis, dandruff, seborrheic dermatitis, rash generalized, rash maculopapular Vascular Disorders: flushing Discontinuations Due to Adverse Reactions Schizophrenia Approximately 11% (22/202) of RISPERDAL CONSTA®-treated patients in the 12-week double- blind, placebo-controlled schizophrenia trial discontinued treatment due to an adverse event, compared with 13% (13/98) who received placebo. The adverse reactions associated with discontinuation in two or more RISPERDAL CONSTA®-treated patients were: agitation (3%), depression (2%), anxiety (1%), and akathisia (1%). Bipolar Disorder In the 24-month double-blind, placebo-controlled treatment period of the trial assessing the efficacy and safety of RISPERDAL CONSTA® when administered as monotherapy for maintenance treatment in patients with bipolar I disorder, 1 (0.6%) of 154 RISPERDAL CONSTA®-treated patients discontinued due to an adverse reaction (hyperglycemia). In the 52-week double-blind phase of the placebo-controlled trial in which RISPERDAL CONSTA® was administered as adjunctive therapy to patients with bipolar disorder in addition to continuing with their treatment as usual, approximately 4% (3/72) of RISPERDAL CONSTA®- treated patients discontinued treatment due to an adverse event, compared with 1.5% (1/67) of placebo-treated patients. Adverse reactions associated with discontinuation in RISPERDAL CONSTA®-treated patients were: hypokinesia (one patient) and tardive dyskinesia (one patient). Dose Dependency of Adverse Reactions in Clinical Trials Extrapyramidal Symptoms: Two methods were used to measure extrapyramidal symptoms (EPS) in the 12-week double-blind, placebo-controlled trial comparing three doses of RISPERDAL CONSTA® (25 mg, 50 mg, and 75 mg) with placebo in patients with schizophrenia, including: (1) the incidence of spontaneous reports of EPS symptoms; and (2) the change from baseline to endpoint on the total score (sum of the subscale scores for parkinsonism, dystonia, and dyskinesia) of the Extrapyramidal Symptom Rating Scale (ESRS). As shown in Table 1, the overall incidence of EPS-related adverse reactions (akathisia, dystonia, parkinsonism, and tremor) in patients treated with 25 mg RISPERDAL CONSTA® was comparable to that of patients treated with placebo; the incidence of EPS-related adverse reactions was higher in patients treated with 50 mg RISPERDAL CONSTA®. The median change from baseline to endpoint in total ESRS score showed no worsening in patients treated with RISPERDAL CONSTA® compared with patients treated with placebo: 0 (placebo group); -1 (25-mg group, significantly less than the placebo group); and 0 (50-mg group). Dystonia Class Effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups. Changes in ECG The electrocardiograms of 202 schizophrenic patients treated with 25 mg or 50 mg RISPERDAL CONSTA® and 98 schizophrenic patients treated with placebo in the 12-week double-blind, placebo-controlled trial were evaluated. Compared with placebo, there were no statistically significant differences in QTc intervals (using Fridericia’s and linear correction factors) during treatment with RISPERDAL CONSTA®. The electrocardiograms of 227 patients with Bipolar I Disorder were evaluated in the 24-month double-blind, placebo-controlled period. There were no clinically relevant differences in QTc intervals (using Fridericia’s and linear correction factors) during treatment with RISPERDAL CONSTA® compared to placebo. The electrocardiograms of 85 patients with bipolar disorder were evaluated in the 52-week double- blind, placebo-controlled trial. There were no statistically significant differences in QTc intervals (using Fridericia’s and linear correction factors) during treatment with RISPERDAL CONSTA® 25 mg, 37.5 mg, or 50 mg when administered as adjunctive treatment in addition to continuing treatment as usual compared to placebo. Pain Assessment and Local Injection Site Reactions The mean intensity of injection pain reported by patients with schizophrenia using a visual analog scale (0 = no pain to 100 = unbearably painful) decreased in all treatment groups from the first to the last injection (placebo: 16.7 to 12.6; 25 mg: 12.0 to 9.0; 50 mg: 18.2 to 11.8). After the sixth injection (Week 10), investigator ratings indicated that 1% of patients treated with 25 mg or 50 mg RISPERDAL CONSTA® experienced redness, swelling, or induration at the injection site. In a separate study to observe local-site tolerability in which RISPERDAL CONSTA® was administered into the deltoid muscle every 2 weeks over a period of 8 weeks, no patient discontinued treatment due to local injection site pain or reaction. Clinician ratings indicated that only mild redness, swelling, or induration at the injection site was observed in subjects treated with 37.5 mg or 50 mg RISPERDAL CONSTA® at 2 hours after deltoid injection. All ratings returned to baseline at the predose assessment of the next injection 2 weeks later. No moderate or severe reactions were observed in any subject. 6.2 Postmarketing Experience The following adverse reactions have been identified during postapproval use of risperidone; because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency: agranulocytosis, alopecia, anaphylactic reaction, angioedema, atrial fibrillation, blood cholesterol increased, blood triglycerides increased, catatonia, diabetes mellitus, diabetic ketoacidosis in patients with impaired glucose metabolism, drug withdrawal syndrome neonatal, dysgeusia, hypoglycemia, hypothermia, ileus, inappropriate antidiuretic hormone secretion, intestinal obstruction, jaundice, mania, pancreatitis, priapism, QT prolongation, sleep apnea syndrome, somnambulism, Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), thrombocytopenia, thrombotic thrombocytopenic purpura, urinary retention, and water intoxication. In addition, the following adverse reactions have been observed during postapproval use of RISPERDAL CONSTA®: cerebrovascular disorders, including cerebrovascular accidents, and diabetes mellitus aggravated. Retinal artery occlusion after injection of RISPERDAL CONSTA® has been reported during postmarketing surveillance. This has been reported in the presence of abnormal arteriovenous anastomosis. Serious injection site reactions including abscess, cellulitis, cyst, hematoma, necrosis, nodule, and ulcer have been reported with RISPERDAL CONSTA® during postmarketing surveillance. Isolated cases required surgical intervention. Very rarely, cases of anaphylactic reaction after injection with RISPERDAL CONSTA® have been reported during postmarketing experience in patients who have previously tolerated oral risperidone. Postmarketing cases of extrapyramidal symptoms (dystonia and dyskinesia) have been reported in patients concomitantly taking methylphenidate and risperidone when there was an increase or decrease in dosage, initiation, or discontinuation of either or both medications. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il
Effects on Driving

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול בסכיזופרניה והפרעות סכיזואפקטיביות בהתקיים כל אלה: א. חולים שאושפזו בעבר ונקלעו לסיכון ממשי של אשפוז חוזר עקב אי הענות לנטילת התכשיר. ב. התחלת הטיפול בתרופה תהיה על פי הוראתו של מנהל מחלקה בבית חולים או מנהל מרפאה שהם רופאים מומחים בפסיכיאטריה או בפסיכיאטריה של הילד והמתבגר.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| סכיזופרניה והפרעות סכיזואפקטיביות |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
15/04/2005
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
15.12.21 - עלון לצרכן אנגלית 15.12.21 - עלון לצרכן עברית 15.12.21 - עלון לצרכן ערבית 08.05.23 - עלון לצרכן עברית 20.10.23 - עלון לצרכן אנגלית 20.10.23 - עלון לצרכן עברית 20.10.23 - עלון לצרכן ערבית 15.05.12 - החמרה לעלון 20.05.14 - החמרה לעלון 20.06.16 - החמרה לעלון 29.01.19 - החמרה לעלון 06.09.20 - החמרה לעלון 21.12.20 - החמרה לעלון 07.04.21 - החמרה לעלון 08.03.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
ריספרדל קונסטה 25 מ"ג