Quest for the right Drug
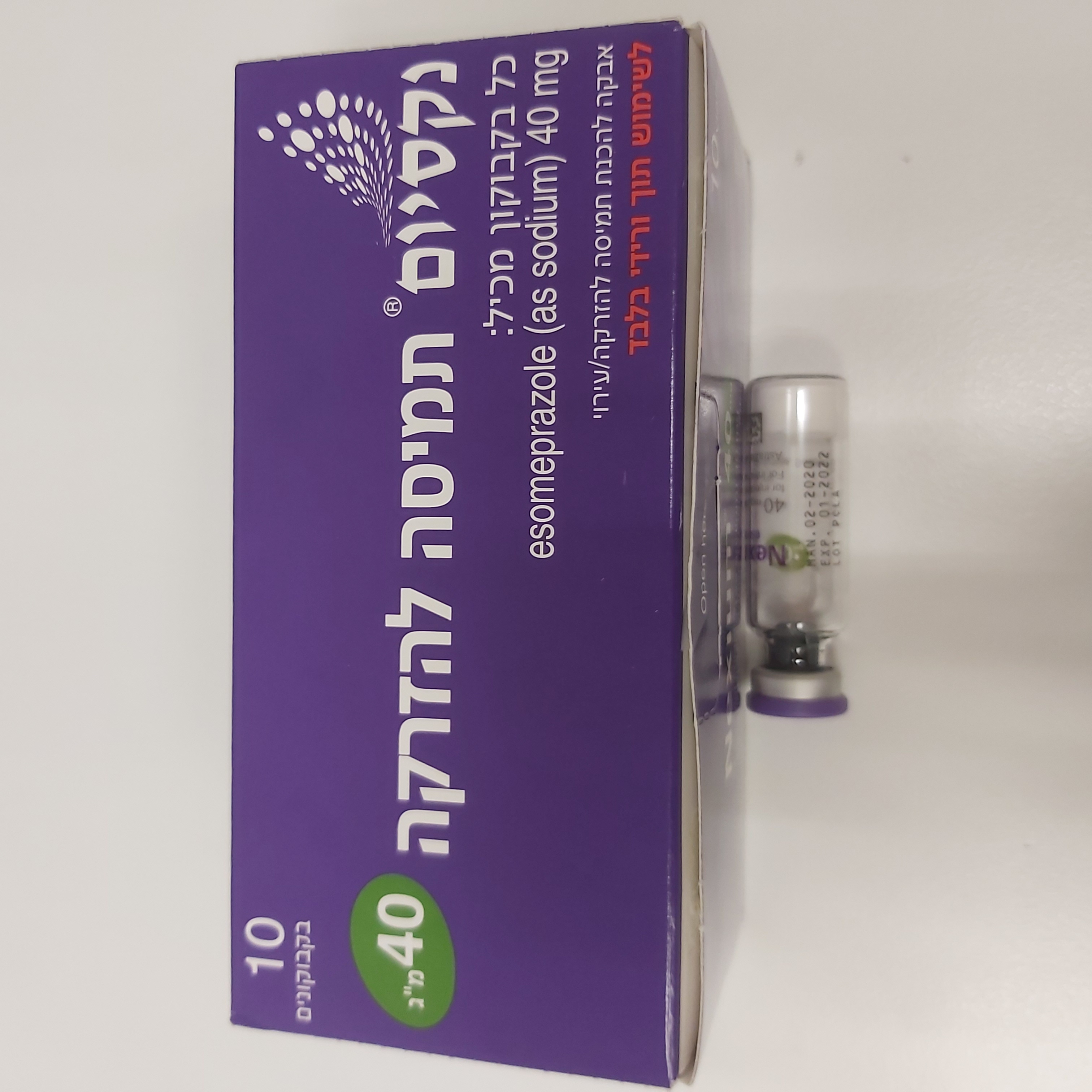
נקסיום אבקה לתמיסה להזרקה/ עירוי 40 מ"ג NEXIUM POWDER FOR SOLUTION FOR INJ/INF 40 MG (ESOMEPRAZOLE AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להמסה להזרקהאינפוזיה : POWDER FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Each vial contains disodium edetate 1.5 mg and sodium hydroxide q.s. for pH adjustment. 6.2 Incompatibilities This medicinal product must not be used with other medicinal products except those mentioned in section 6.6. 6.3 Shelf-life The expiry date is indicated on the packaging materials. Shelf-life reconstitution Chemical and physical in-use stability has been demonstrated for 12 hours at 25ºC. From a microbiological point of view, the product should be used immediately. 6.4 Special precautions for storage Store in the original package, in order to protect from light. Vials can however be stored exposed to normal in-door light outside the box for up to 24 hours. Do not store above 25ºC. 6.5 Nature and contents of container 5 mL vial made of colourless borosilicate glass, type 1. Stopper made of grey bromobutyl rubber, cap made of aluminium and a plastic flip-off seal. Pack sizes: 10 vials. 6.6 Instructions for use, handling and disposal The reconstituted solution should be inspected visually for particulate matter and discoloration prior to administration. Only clear solution should be used. For single use only. If the entire reconstituted content of the vial is not required, any unused solution should be discarded in accordance with local requirements. Injection 40 mg A solution for injection (8 mg/ml) is prepared by adding 5 mL of 0.9% sodium chloride for intravenous use to the esomeprazole 40 mg vial. The reconstituted solution for injection is clear and colourless to very slightly yellow. Infusion 40 mg A solution for infusion is prepared by dissolving the content of one vial with esomeprazole 40 mg in up to 100 mL 0.9% sodium chloride for intravenous use. Infusion 80 mg A solution for infusion is prepared by dissolving the content of two vials of esomeprazole 40 mg in up to 100 ml of 0.9% sodium chloride for intravenous use. The reconstituted solution for infusion is clear and colourless to very slightly yellow. 7. MANUFACTURER: AstraZeneca AB, S-151 85 Södertälje, Sweden

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לרופא
19.07.21 - עלון לרופאלתרופה במאגר משרד הבריאות
נקסיום אבקה לתמיסה להזרקה/ עירוי 40 מ"ג