Quest for the right Drug
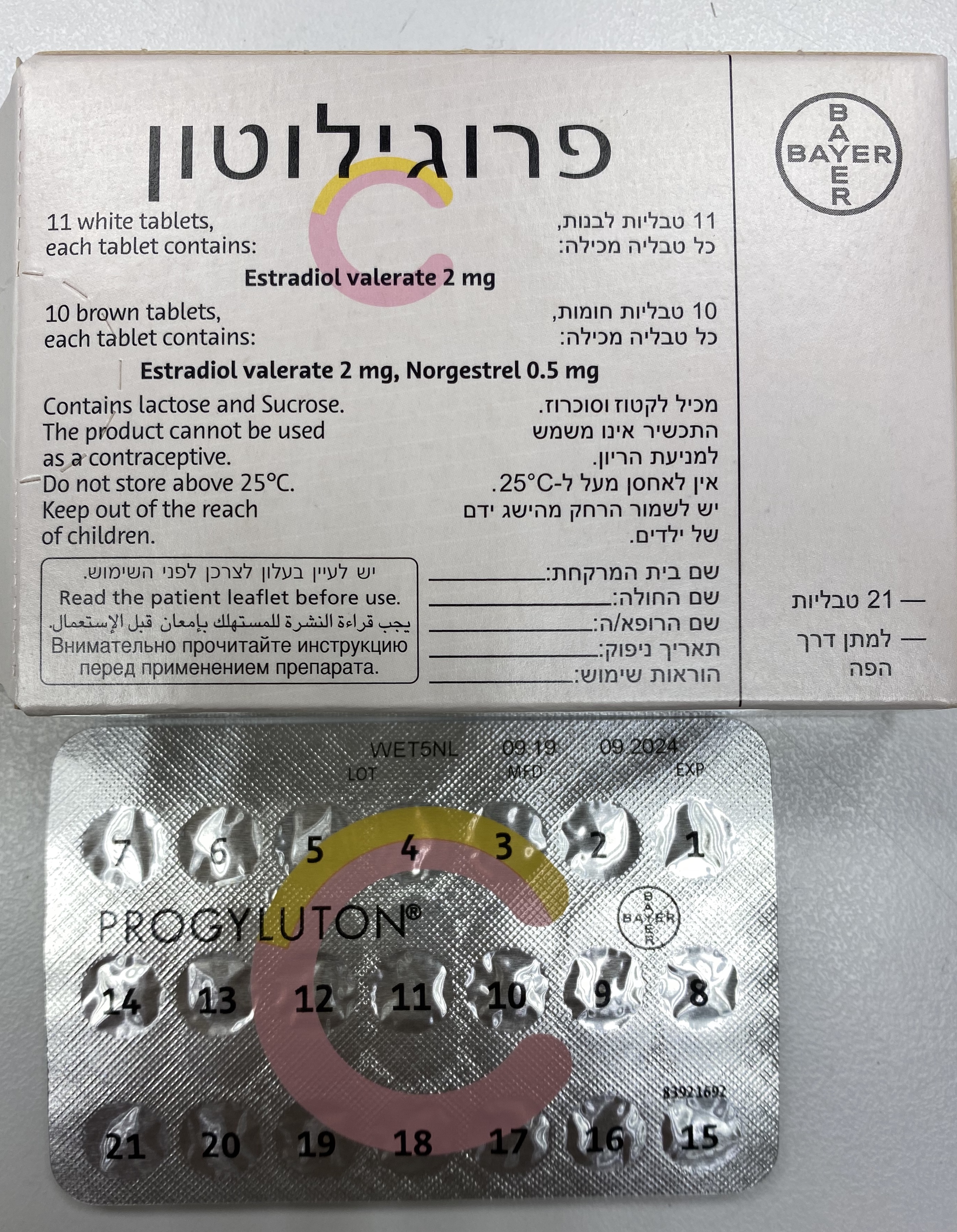
פרוגילוטון PROGYLUTON (ESTRADIOL VALERATE, NORGESTREL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The serious undesirable effects linked to the use of an HRT are also described in the section “Special warnings and special precautions for use” (q.v.). Page 10 of 16 The following lists the undesirable effects by organ system and frequency, observed under HRT preparations. A connection with Progyluton cannot be corroborated, nor can it be ruled out. The frequency brackets are defined as follows: common ≥1/100 and <1/10; uncommon ≥1/1,000 and <1/100; rare ≥1/10,000 and <1/1,000); unknown ( cannot be estimated from the available data) Benign, malignant and unspecific growths (including cysts and polyps) Uncommon: mammary carcinoma. Unknown: endometrial carcinoma. Diseases of the immune system Uncommon: hypersensitivity reactions. Metabolic and nutritional disorders Common: weight gain. Unknown: weight loss. Psychiatric diseases Common: mood swings, depression. Uncommon: changes in libido*, nervousness. Rare: anxiety. * Both decreased and increased libido have been reported. Diseases of the nervous system Common: headache. Uncommon: sleep disorders, dizziness, migraine. Eye diseases Uncommon: visual impairments. Heart disease Uncommon: palpitations, increased blood pressure, arterial thromboembolic events (e.g. myocardial infarction, apoplexy). Vascular disease Uncommon: venous thromboembolic events (e.g., deep vein thrombosis, pulmonary embolism) Diseases of the gastrointestinal tract Common: flatulence, stomach pain, nausea, dyspepsia. Uncommon: vomiting. Liver and gallbladder disorders Uncommon: abnormal liver function tests. Very rare: cholestatic jaundice. Page 11 of 16 Unknown: cholelithiasis (and other gallbladder diseases). Diseases of the skin and subcutaneous tissue Common: skin rash, pruritus. Uncommon: acne, hirsutism, alopecia, urticaria. Unknown: chloasma, erythema nodosum, erythema multiforme, vascular purpura. Skeletomuscular, connective tissue and bone diseases Common: back pain. Uncommon: muscle cramps. Diseases of the sex organs and of the mammary glands Very common: feeling of tightness in the breasts, breast pain, bleeding anomalies (menorrhagia, metrorrhagia, spotting, etc.) Common: abdominal pain, vaginal discharge, increased size of uterine myomas, enlargement of the breasts. Rare: dysmenorrhea, premenstrual syndrome. Unknown: endometrial hyperplasia. General diseases Common: peripheral edema, asthenia. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/

שימוש לפי פנקס קופ''ח כללית 1994
Deficiency symptoms in the pre- & postmenopause, after oophorectomy or radiological castration for non-carcinomatous diseases, functional amenorrhea, irregularities of the menstrual cycle
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
