Quest for the right Drug
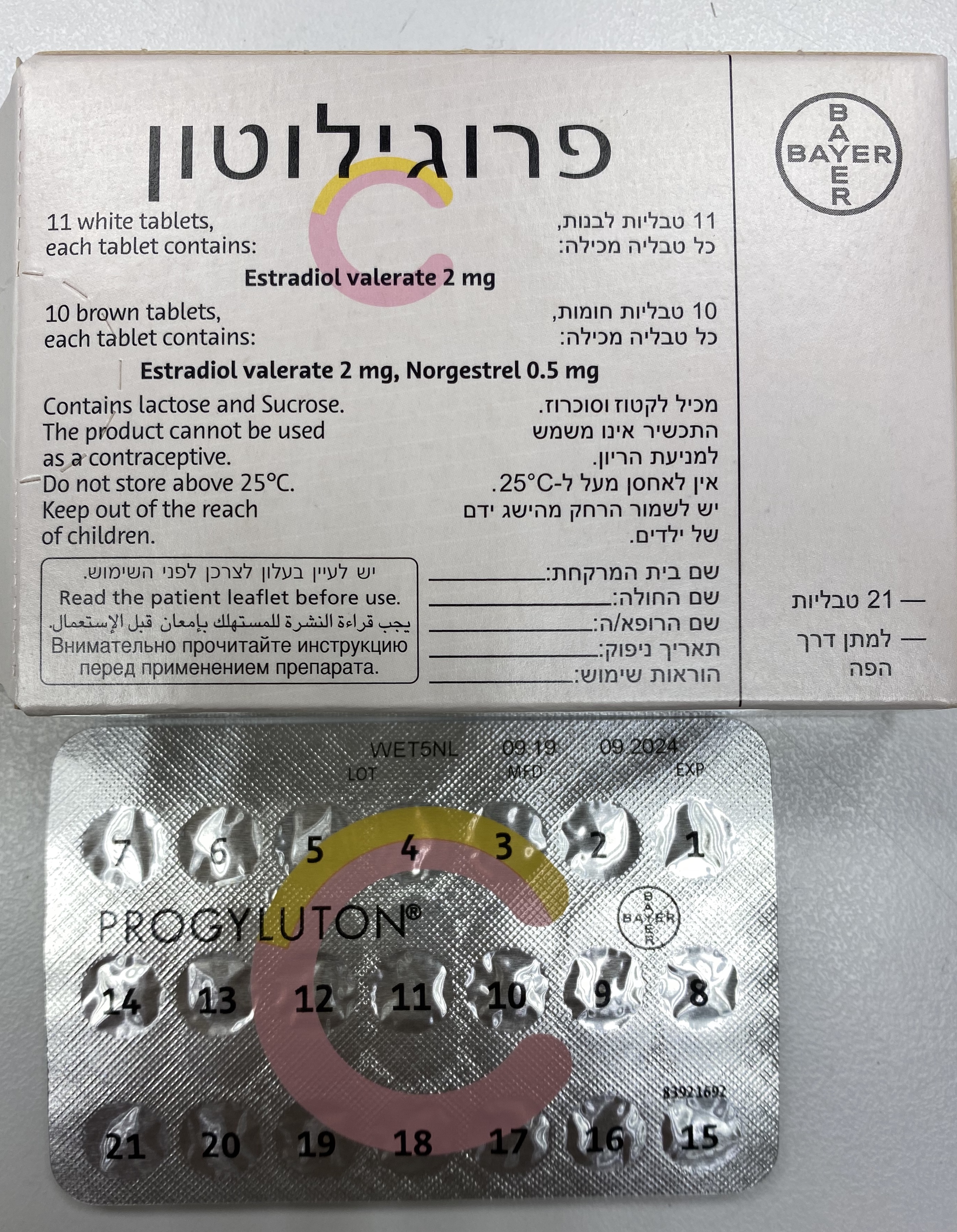
פרוגילוטון PROGYLUTON (ESTRADIOL VALERATE, NORGESTREL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties ATC code: G03FB01 Mode of action Page 12 of 16 Progyluton is a two-step HRT preparation for after the menopause and for regulating the cycle in younger women Estradiol After oral administration the active substance estradiol valerate is rapidly released. Estradiol, which is primarily produced by the ovarian follicle in women from the menarche to the menopause, is the most effective estrogen at the receptor level (e.g., uterus, vagina, urethra, breast, hypothalamus, pituitary, osteoblasts and liver). In many women the failure of ovarian estrogen leads to vasomotor and thermoregulatory instability (hot flushes), disturbed sleep, depressive moods and an increasing atrophy of the urogenital system. These disturbances can be largely rectified by estrogen replacement. On the other hand, Progyluton can only improve depressive moods, if they occur in the context of vasomotor symptoms. Estrogen replacement at doses that effect an improvement in the menopausal complaints also has a strong excitatory effect on mitosis and the proliferation of the endometrium. Estrogen monotherapy increases the incidence of endometrial hyperplasia and the risk of developing endometrial cancer. In Progyluton estradiol valerate is cyclically combined with norgestrel, thus largely obviating this risk. Norgestrel Norgestrel is a progestogen which in general mimics the biological effects of the endogenously produced progestogen progesterone: Progesterone affects all tissues that contain estrogen receptors, it induces protein synthesis and at the same time reduces the number of estrogen and progesterone receptors, thus limiting any excessive stimulation of growth in the target organs caused by the estrogen. The main target organs of the progestogens include the uterus in which the secretory transformation of the endometrium proliferated under estrogen influence is induced by its effect. When the progestogen concentration falls, the endometrium previously proliferated by the estrogen is then shed. The additional administration of a progestogen on 10 to 14 days (preferably 12) during each cycle together with a continuous estrogen therapy largely prevents the overstimulation of the endometrium, which would otherwise happen with an estrogen monotherapy. This considerably reduces the incidence of hyperplasia, which can lead to bleeding irregularities and to endometrial cancer. Progyluton is especially suitable for women in the perimenopause: It overcomes the typical subjective complaints and regularizes the cycle. At a dosage of 2 mg estradiol valerate has only a very small central inhibitory effect. As a result there is in general no ovulation inhibition during the administration of Progyluton, and the body’s own hormone production is scarcely impaired. For this reason the preparation may also be used in specific circumstances for stabilizing and regularizing the cycle in younger women (“Special warnings and special precautions for use”). Page 13 of 16
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Estradiol valerate Absorption After oral administration, estradiol valerate is rapidly and completely absorbed. The steroid ester is split into estradiol and valeric acid as soon as it reaches the intestinal wall and during its first passage through the liver. Because of the first-pass metabolism, the bioavailability of estradiol is only approximately 3%. As a rule, the maximum estradiol concentrations (approx. 30 pg/mL) in plasma are attained 4-9 hours after taking one tablet. Between the treatment phase with estradiol valerate alone and that the treatment phase in combination with norgestrel, the estrogen levels show no relevant differences. Compared with taking the tablet on an empty stomach, taking it with food has no influence on the bioavailability of estradiol. Steady state Estradiol serum levels have been observed to be roughly twice as high after repeated administration when compared to a single dose. On average, the steady state concentration is Cmin 30 pg/mL and Cmax 60 pg/mL. Distribution In plasma approx. 60 % of the estradiol is bound to albumin and just short of 40 % to SHBG. Only 1-1.5 % of the estradiol is freely available in the plasma. The apparent distribution volume of estradiol after a single intravenous dose is approx. 1 L/kg. Estradiol passes the placental barrier. Only a very small amount of estradiol and its metabolites find their way into the mother's milk. Metabolism Following the ester splitting of estradiol valerate, an extensive metabolism of the estradiol, involving CYP3A4, takes place, producing primarily estrone, estriol and estrone sulfate, and this follows the biotransformation path of the endogenous estradiol. The main active metabolite, estrone, achieves plasma levels that are approximately 8 times higher compared with estradiol, and estrone sulfate achieves plasma levels that are around 150 times higher. The metabolic clearance rate for estradiol is about 10-30 mL/min/kg. Elimination Estradiol metabolites are conjugated to around 90 % with glucuronide or sulfate and are excreted via the urine with a half-life of approx. one day. Only approx. 10 % of the metabolites are excreted in the feces and are subject to an enterohepatic circulation. 2-3 days after therapy has stopped, the levels of estradiol return to those before therapy started. Norgestrel Absorption Following oral administration, norgestrel is quickly and completely absorbed. The active component of the racemate norgestrel is levonorgestrel, which is fully bioavailable. As a rule Page 14 of 16 plasma levels of levonorgestrel peak at 7-8 ng/mL as early as 1-1.5 h after a single dose of Progyluton. Steady state After repeated administration, elevated trough levels of approximately 1 ng/mL were measured. In the case of administration of the estrogen-progestogen combination, however, the exposure (AUC) does not differ in a relevant manner between the steady state and a single dose. Distribution Only approximately 1.3% of the overall serum concentration of levonorgestrel is in the form of a free steroid, approximately 64% is bound specifically to SHBG and approximately 35% is bound nonspecifically to albumin. The relative proportions of free levonorgestrel, levonorgestrel bound to albumin and levonorgestrel bound to SHBG are heavily dependent on the concentration of SHBG in the plasma. The concentration of SHBG peaks at the end of the estrogen monophase of the Progyluton therapy cycle and then falls to a trough by the end of the combination phase. The estradiol-induced increase in SHBG concentration results in an increase in the proportion bound to SHBG and in a reduction in the free proportion. Approximately 0.1% of the applied dose passes into the mother's milk. Metabolism Levonorgestrel is extensively metabolized in the liver. The major metabolic degradation pathways are reduction of the Δ4-3-oxo group and hydroxylations at the 2α, 1β, and 16β positions, followed by conjugation. CYP3A4 is involved as the main enzyme in the oxidative metabolism of levonorgestrel. However, available in vitro data suggest that CYP-mediated biotransformation reactions may be of secondary importance in the metabolism of levonorgestrel compared with reduction and conjugation. Pharmacologically active metabolites of levonorgestrel are unknown. Elimination Elimination of levonorgestrel is in two stages. The clearance rate is roughly 1 mL/min/kg. The metabolites are excreted with a half-life of approx. 1 day in roughly equal shares in the urine and bile. Kinetics of special patient groups Impaired kidney function: The pharmacokinetics of Progyluton in patients with renal insufficiency have not been investigated. Impaired liver function: The pharmacokinetics of Progyluton in patients with hepatic insufficiency have not been investigated. However, it is known that the metabolic breakdown of estrogens and progestogens is slowed in the case of liver function disorders (see also "Warnings and precautions").

שימוש לפי פנקס קופ''ח כללית 1994
Deficiency symptoms in the pre- & postmenopause, after oophorectomy or radiological castration for non-carcinomatous diseases, functional amenorrhea, irregularities of the menstrual cycle
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
