Quest for the right Drug
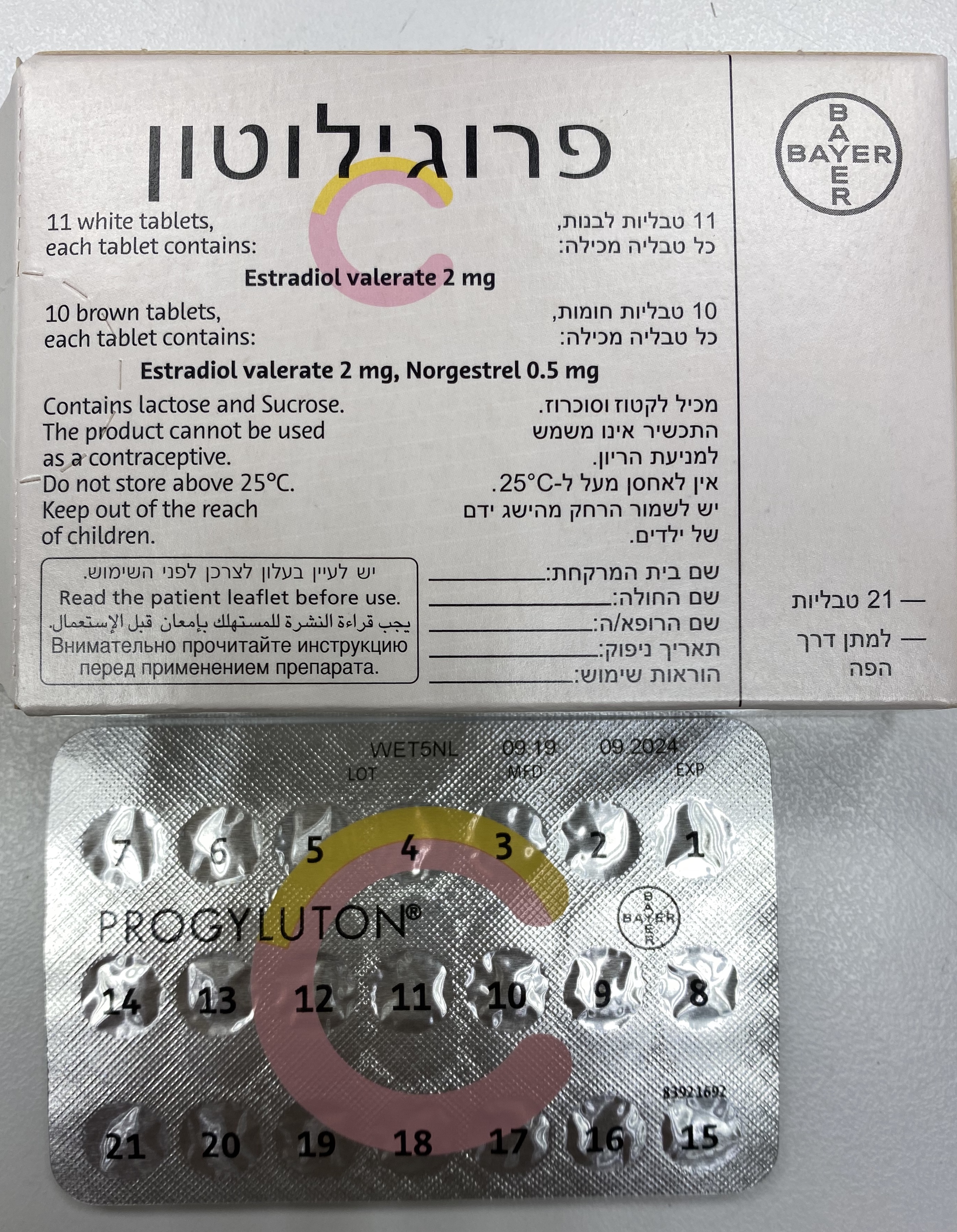
פרוגילוטון PROGYLUTON (ESTRADIOL VALERATE, NORGESTREL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Progyluton is a cyclic HRT product. One tablet is to be taken orally once a day for 21 days, followed by a 7-day tablet free interval. Therefore each new pack is started after a 28 day cycle. The white tablets should be taken from days 1 to 11 followed by the brown tablets from days 12 to 21. It is recommended that the tablets are taken at the same time every day. For initiation and continuation of treatment of peri- and post-menopausal symptoms the lowest effective dose for the shortest duration (see also 'Special warnings and special precautions for use') should be used. For women still having periods, the first tablet should be taken on the 5th day of their menstrual period. If menstruation has stopped, or is infrequent or sporadic, then the first tablet can be taken any time. If the patient is being transferred from a continuous HRT product, the patient may start Progyluton on any convenient day. For those transferring from a cyclic or sequential product, Progyluton should be started following completion of the previous regimen. If a tablet is missed, it should be taken as soon as possible, unless it is more than 12 hours late. In this case the missed tablet should be left in the pack and the next tablet taken at the right time. Missing a dose may result in breakthrough bleeding or spotting. Unless there is a previous diagnosis of endometriosis, it is not recommended that progestogen- containing HRT be given to hysterectomised women. Children and adolescents There are no data available on the safety and efficacy in children and adolescents under 18 years of age. Progyluton is not indicated for use in children and adolescents Geriatric patients There are no data suggesting a need for dosage adjustment in women aged 65 years or older (see section 4.4 Special warnings and precautions for use) Patients with renal impairment Progyluton has not been specifically studied in renally impaired patients. Available data do not suggest a need for dosage adjustment in this patient population Hepatic insufficiency Progyluton has not been tested in patients with hepatic insufficiency. Progyluton is contraindicated in women with serious hepatic conditions.

שימוש לפי פנקס קופ''ח כללית 1994
Deficiency symptoms in the pre- & postmenopause, after oophorectomy or radiological castration for non-carcinomatous diseases, functional amenorrhea, irregularities of the menstrual cycle
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
