Quest for the right Drug
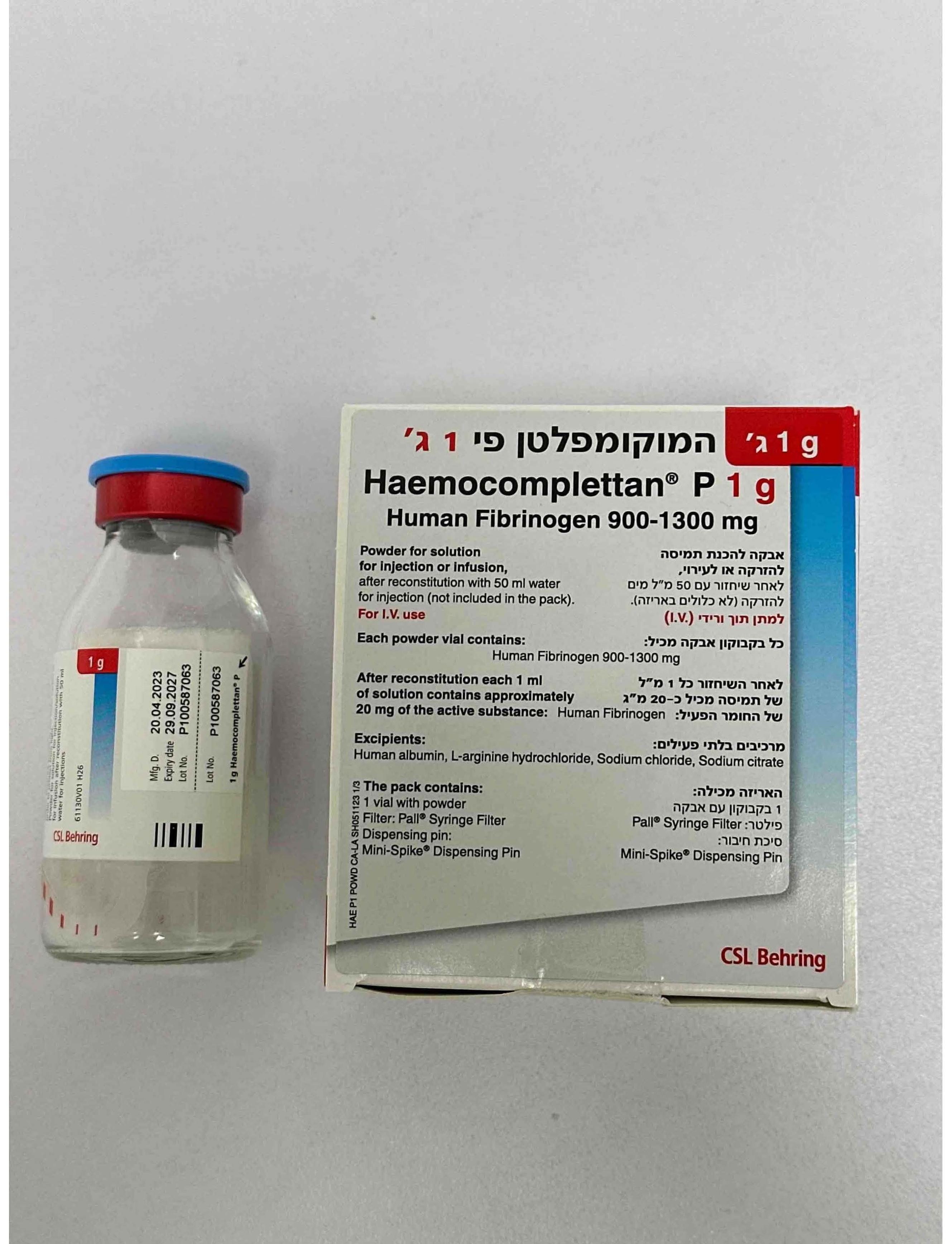
המוקומפלטן פי 1 ג' HAEMOCOMPLETTAN P 1 G (HUMAN FIBRINOGEN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להמסה להזרקהאינפוזיה : POWDER FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Human albumin, L-arginine hydrochloride, sodium chloride, sodium citrate 6.2 Incompatibilities This medicinal product must not be mixed with other medicinal products, diluents, or solvents except those mentioned in section 6.6. A standard infusion set is recommended for intravenous application of the reconstituted solution at room temperature. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. The physico-chemical stability for the reconstituted product has been demonstrated for 8 hours at room temperature (max. +25 °C). From a microbiological point of view the product should be used immediately following reconstitution. If the reconstituted product is not administered immediately, storage shall not exceed 8 hours below 25 °C. The reconstituted product should not be stored in the refrigerator. 6.4 Special precautions for storage Store in a refrigerator (2 0 C – 8 0C). Do not freeze! Keep the vial in the closed outer carton, in order to protect from light. Stability for the reconstituted product see section 6.3. 6.5 Nature and contents of container Vial of colourless glass (Type II Ph. Eur.) closed with a latex-free stopper (bromobutyl rubber), and sealed with an aluminium / plastic crimp cap. Pack with 1 or 2 g (Figure 1) 1. One vial containing 1 or 2 g human fibrinogen 2. Filter: Pall® Syringe Filter 3. Dispensing pin: Mini-Spike® Dispensing Pin Figure 1 6.6 Special precautions for disposal and other handling General instructions • Reconstitution and withdrawal should be carried out under aseptic conditions. • Reconstituted product should be inspected visually for particulate matter and discoloration prior to administration. • The solution should be almost colourless to yellowish, clear to slightly opalescent and of neutral pH. Do not use solutions that are cloudy or contain residues (deposits/particles). Reconstitution • Warm both the solvent and the powder in unopened vials to room or body temperature (not above 37 °C). • Haemocomplettan should be reconstituted with water for injections (50 ml for 1 g and 100 ml for 2 g, respectively, not included). • Wash hands or use gloves before reconstituting the product. • Remove the cap from the Haemocomplettan vial to expose the central portion of the infusion stopper. • Treat the surface of the infusion stopper with antiseptic solution and allow it to dry. • Transfer the solvent with an appropriate transfer device into the infusion vial. Ensure to push straight down centrally through the infusion stopper. Transfer the solvent completely. The powder should be completely wet. • Gently swirl the vial until the powder is reconstituted and the solution is ready for administration. Avoid strong shaking which causes formation of foam. The powder should be completely reconstituted within max. 15 minutes (generally within 5 to 10 minutes). • Open the plastic blister containing the dispensing pin (Mini-Spike® Dispensing Pin) provided with Haemocomplettan (Figure 2). Figure 2 • Take the provided dispensing pin and insert it into the stopper of the vial with the reconstituted product (Figure 3). Figure 3 • After the dispensing pin is inserted, remove the cap. After the cap is removed, do not touch the exposed surface. • Open the blister with the filter (Pall® Syringe Filter) provided with Haemocomplettan (Figure 4). Figure 4 • Screw the syringe onto the filter (Figure 5). Figure 5 • Screw the syringe with the mounted filter onto the dispensing pin (Figure 6). Figure 6 • Draw the reconstituted product into the syringe (Figure 7). Figure 7 • When completed, remove the filter, dispensing pin and empty vial from the syringe, dispose of properly, and proceed with administration as usual. • Reconstituted product should be administered immediately by a separate injection / infusion line (see section 6.3). • Take care that no blood enters syringes filled with product. Any unused product or waste material should be disposed of in accordance with local requirements.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
