Quest for the right Drug
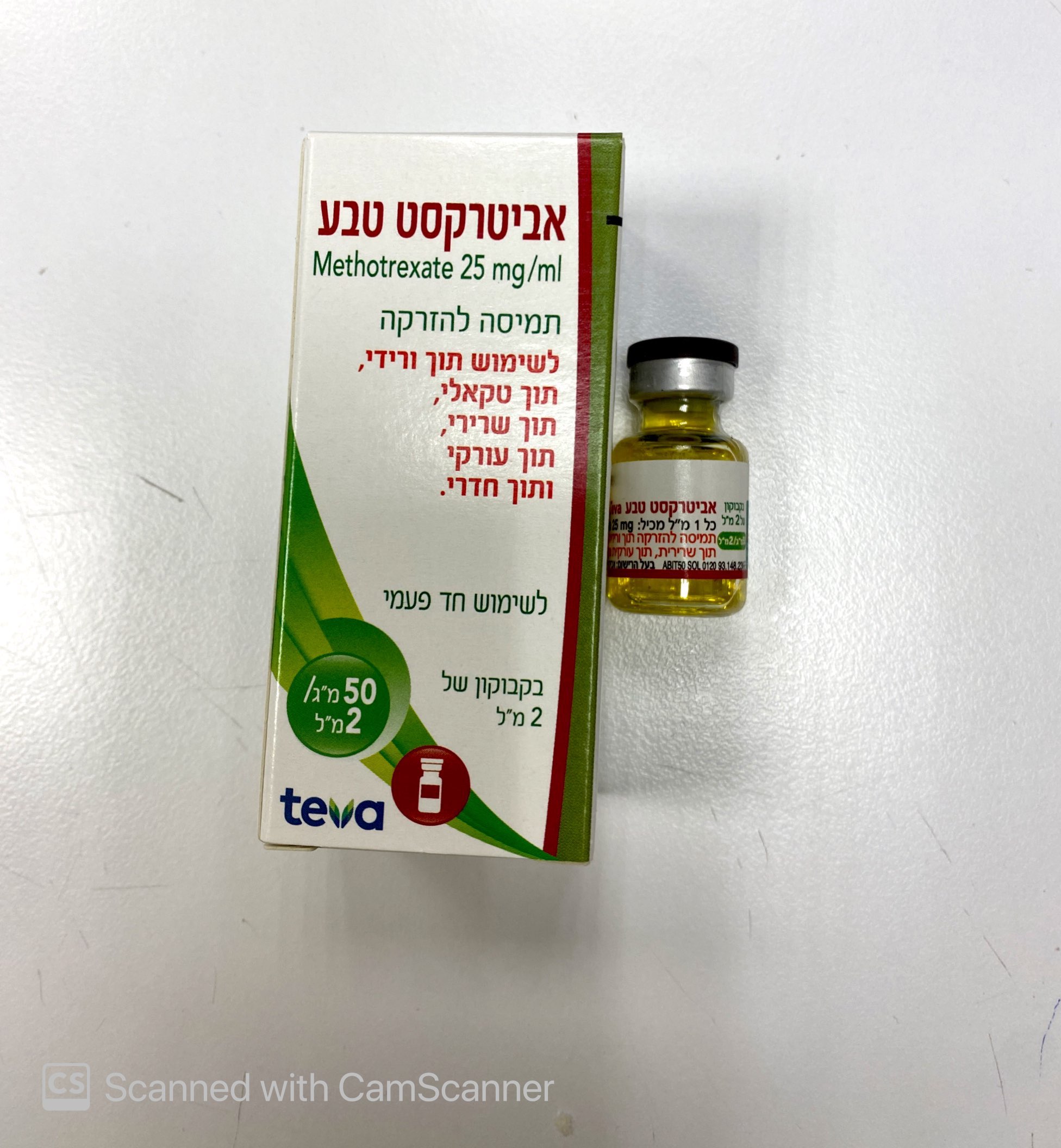
אביטרקסט טבע ABITREXATE TEVA (METHOTREXATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי, תוך-שדרתי, תוך-עורקי, תוך חדרי : I.M, I.V, INTRATHECAL, INTRA-ARTERIAL, INTRA VENTRICULAR
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Contraindications : התוויות נגד
CONTRAINDICATIONS Abitrexate Teva is contraindicated: • In patients who are hypersensitive to the active substance or to any of the excipients listed in section DOSAGE FORMS, COMPOSITION AND PACKAGING. • In patients with severe renal impairment including end stage renal disease with and without dialysis (see WARNINGS AND PRECAUTIONS - Renal, Special populations and DOSAGE AND ADMINISTRATION). • In pregnant patients with psoriasis or rheumatoid arthritis and should be used in the treatment of neoplastic diseases only when the potential benefit outweighs the risk to the fetus. • In women of childbearing potential until pregnancy is excluded. • In nursing mothers. • In patients with psoriasis or rheumatoid arthritis with alcoholism, alcoholic liver disease or other chronic liver disease. • In patients with psoriasis or rheumatoid arthritis who have overt or laboratory evidence of immunodeficiency syndromes. • In patients with psoriasis or rheumatoid arthritis who have pre-existing blood dyscrasias, such as bone marrow hypoplasia, leucopenia, thrombocytopenia or significant anemia. • With nitrous oxide anesthesia (see WARNINGS AND PRECAUTIONS: Renal and DRUG INTERACTIONS: Drug-Drug Interactions). WARNINGS AND PRECAUTIONS Serious Warnings and Precautions • Abitrexate Teva should be used only by physicians whose knowledge and experience includes the use of antimetabolite therapy because of the possibility of serious toxic reactions (see WARNINGS AND PRECAUTIONS: General). • Methotrexate has been reported to cause fetal death and/or congenital anomalies (see Special Populations: Pregnant Women section below). Therefore, use is contraindicated for women of childbearing potential until pregnancy is excluded and pregnant patients with psoriasis or rheumatoid arthritis (see CONTRAINDICATIONS). General Fatal toxicities related to inadvertent daily rather than weekly dosing have been reported, particularly in elderly patients. It should be emphasized to the patient that the recommended dose is taken weekly for rheumatoid arthritis and psoriasis, and that daily use of the weekly recommended dose has led to fatal toxicity. Fatal toxicities related to intravenous dosing miscalculation have been reported. Special attention must be given to dose calculation. Because of the possibility of serious toxic reactions (which can be fatal), methotrexate should be used only in neoplastic diseases (as indicated), or in patients with severe, recalcitrant, disabling psoriasis or severe rheumatoid arthritis that are not adequately responsive to other forms of therapy. The patient should be informed by the physician of the risks involved and should be under a physician’s constant supervision. The use of methotrexate high-dose regimens recommended for osteosarcoma requires meticulous care (see DOSAGE AND ADMINISTRATION). High dosage regimens for other neoplastic diseases are investigational and a therapeutic advantage has not been established. Toxic effects may be related in frequency and severity to dose or frequency of administration but have been seen at all doses. Because they can occur at any time during therapy, it is necessary to follow patients on methotrexate closely. Most adverse reactions are reversible if detected early. When such reactions do occur, the drug should be reduced in dosage or discontinued and appropriate corrective measures should be taken. If necessary, this could include the use of leucovorin calcium and/or acute, intermittent hemodialysis with a high-flux dialyzer (see OVERDOSAGE). If methotrexate is re-instituted, it should be carried out with caution, with adequate consideration of further need for the drug and with increased alertness as to possible recurrence of toxicity. Methotrexate may induce "tumour lysis syndrome" in patients with rapidly growing tumours. Appropriate supportive and pharmacologic measures may prevent or alleviate this complication. Methotrexate exits slowly from third space compartments (e.g., pleural effusions or ascites). This results in a prolonged terminal plasma half-life and unexpected toxicity. In patients with significant third space accumulations, it is advisable to evacuate the fluid before treatment and to monitor plasma methotrexate levels. Unexpectedly severe (sometimes fatal) bone marrow suppression, aplastic anemia and gastrointestinal toxicity have been reported with concomitant administration of methotrexate (usually in high dosage) along with non-steroidal anti-inflammatory drugs (NSAIDs) (see DRUG INTERACTIONS). Bone marrow and mucosal toxicity depend on dose and duration of exposure of high levels (>2x10-8 mol/L (0.02 micromolar)) of methotrexate. Since the critical time factor has been defined for these organs as being 42 hours in humans, this has the following implications: • when high doses of methotrexate are employed (>1g/m2), drug levels in serum should be monitored • when drug levels exceeding 2x10-8 mol/L (0.02 micromolar) for >42 hours may forecast significant toxicity • when toxicity can be minimized by appropriate administration of Leucovorin Calcium • when high-dose methotrexate (HDMTX) is employed, it is imperative to alkalinise the urine in order to prevent crystallisation of methotrexate and its 7-hydroxy metabolite in the urine, which may lead to acute renal failure. Methotrexate given concomitantly with radiotherapy may increase the risk of soft tissue necrosis and osteonecrosis. Methotrexate should be used with extreme caution in the presence of debility. The use of methotrexate high-dose regimens (>500 mg/m2) recommended for osteosarcoma requires meticulous care. High-dosing regimens for other neoplastic diseases are investigational and a therapeutic advantage has not been established. Drug Interactions with Proton Pump Inhibitors (PPI) Use caution when administering high-dose methotrexate to patients receiving proton pump inhibitor (PPI) therapy. Case reports and published population pharmacokinetic studies suggest that concomitant use of some PPIs, such as omeprazole, esomeprazole, and pantoprazole, with methotrexate (primarily at high dose), may elevate and prolong serum levels of methotrexate and/or its metabolite hydromethotrexate, possibly leading to methotrexate toxicities. In two of these cases, delayed methotrexate elimination was observed when high-dose methotrexate was co- administered with PPIs, but was not observed when methotrexate was co-administered with ranitidine. However, no formal drug interaction studies of methotrexate with ranitidine have been conducted. Carcinogenesis and Mutagenesis Malignant lymphomas may occur in patients receiving low-dose methotrexate. These lymphomas may regress following withdrawal of methotrexate without requiring treatment. No controlled human data exist regarding the risk of neoplasia with methotrexate. Methotrexate has been evaluated in a number of animal studies for carcinogenic potential with inconclusive results. Although there is evidence that methotrexate causes chromosomal damage to animal somatic cells and human bone marrow cells, the clinical significance remains uncertain. Assessment of the carcinogenic potential of methotrexate is complicated by conflicting evidence of an increased risk of certain tumours in rheumatoid arthritis. Benefit should be weighed against this potential risk before using methotrexate alone or in combination with other drugs, especially in children or young adults. (See TOXICOLOGY). Gastrointestinal If vomiting, diarrhea, or stomatitis occurs, resulting in dehydration, methotrexate should be discontinued until recovery occurs. Diarrhea and ulcerative stomatitis require interruption of therapy; otherwise, hemorrhagic enteritis and death from intestinal perforation may occur. methotrexate should be used with extreme caution in the presence of peptic ulcer disease or ulcerative colitis. Use caution when administering high-dose methotrexate to patients receiving proton pump inhibitor (PPI) therapy as concomitant use of some PPIs, such as omeprazole, esomeprazole, and pantoprazole, with methotrexate (primarily at high dose), may elevate and prolong serum levels of methotrexate and/or its metabolite hydromethotrexate, possibly leading to methotrexate toxicities (see DRUG INTERACTIONS: Drug-Drug Interactions). Hematologic Methotrexate should be used with caution in patients with impaired bone marrow function and previous or concomitant wide field radiotherapy. Methotrexate may produce marked bone marrow depression with resultant anemia, aplastic anemia, pancytopenia, leucopenia, neutropenia, and/or thrombocytopenia. In controlled clinical trials in rheumatoid arthritis (n=128), leucopenia (WBC <3000/mm3) was seen in 2 patients, thrombocytopenia (platelets <100,000/mm3) in 6 patients, and pancytopenia in 2 patients. The nadir of circulating leukocytes, neutrophils and platelets usually occurs between 5 and 13 days after an I.V. bolus dose (with recovery between 14 to 28 days). Leukocytes and neutrophils may occasionally show two depressions, the first occurring in 4-7 days and a second nadir after 12-21 days, followed by recovery. Clinical sequel such as fever, infections and hemorrhage from various sites may be expected. In psoriasis and rheumatoid arthritis, methotrexate should be stopped immediately if there is a significant drop in blood counts. In the treatment of neoplastic diseases, methotrexate should be continued only if the potential benefit warrants the risk of severe myelosuppression. Patients with profound granulocytopenia and fever should be evaluated immediately and usually require parenteral broad-spectrum antibiotic therapy. Hepatic/Biliary/Pancreatic Methotrexate has the potential for acute (elevated transaminases) and chronic (fibrosis and cirrhosis) hepatotoxicity. Acutely, liver enzyme elevations are frequently seen after methotrexate administration and are usually not a reason for modification of methotrexate therapy. Liver enzyme elevations are usually transient and asymptomatic, and also do not appear predictive of subsequent hepatic disease. Persistent liver abnormalities, and/or decrease of serum albumin may be indicators of serious liver toxicity. Chronic toxicity is potentially fatal; it generally has occurred after prolonged use (generally two years or more) and after a total cumulative dose of at least 1.5 grams. Liver biopsy after sustained use often shows histologic changes, and fibrosis and cirrhosis have been reported; these latter lesions may not be preceded by symptoms or abnormal liver function tests in the psoriasis population. Periodic liver biopsies are usually recommended for psoriatic patients who are under long-term treatment. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis in the rheumatoid arthritis population. In studies in psoriatic patients, hepatotoxicity appeared to be a function of total cumulative dose and appeared to be enhanced by alcoholism, obesity, diabetes and advanced age. An accurate incidence rate has not been determined; the rate of progression and reversibility of lesions is not known. Special caution is indicated in the presence of pre-existing liver damage or impaired hepatic function. Methotrexate has caused reactivation or worsening of hepatitis B and C infections, in some cases resulting in death. Some cases of hepatitis B reactivation have occurred after discontinuation of methotrexate. Prior to treatment with methotrexate, clinical and laboratory evaluation should be performed to evaluate preexisting hepatitis virus B and hepatitis virus C infection. Methotrexate is not recommended for patients with active or chronic hepatitis B or C infection. In psoriasis, liver damage and function tests, including serum albumin and prothrombin time, should be performed several times prior to dosing, but are often normal in the face of developing fibrosis or cirrhosis. These lesions may be detectable only by biopsy. The usual recommendation is to obtain a liver biopsy: 1) before the start of therapy or shortly after initiation of therapy (4-8 weeks); 2) after a total cumulative dose of 1.5 grams; and 3) after each additional 1.0 to 1.5 grams. Moderate fibrosis or any cirrhosis normally leads to discontinuation of the drug; mild fibrosis normally suggests a repeat biopsy in 6 months. Milder histologic findings such as fatty change and low-grade portal inflammation are relatively common pre-therapy. Although these mild changes are usually not a reason to avoid or discontinue methotrexate therapy, the drug should be used with caution. Clinical experience with liver disease in rheumatoid arthritis is limited, but the same risk factors would be anticipated. Liver function tests are also usually not reliable predictors of histological changes in this population. In rheumatoid arthritis, advanced age at first use of methotrexate and increasing duration of therapy have been reported as risk factors for hepatotoxicity. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis in the rheumatoid population. Liver function tests should be performed at baseline and at 4-8 week intervals in patients receiving methotrexate for rheumatoid arthritis. Pretreatment liver biopsy should be performed for patients with a history of excessive alcohol consumption, persistently abnormal baseline liver function test values, or chronic hepatitis B or C infection. During therapy, liver biopsy should be performed if there are persistent liver function test abnormalities, or there is a decrease in serum albumin below the normal range (in the setting of well controlled rheumatoid arthritis). If the results of a liver biopsy show mild changes (Roenigk grades I, II, IIIa), methotrexate may be continued and the patient monitored according to the recommendations listed above. Methotrexate should be discontinued in any patient who displays persistently abnormal liver function tests and refuses liver biopsy, or in any patient whose liver biopsy shows moderate to severe changes (Roenigk grade IIIb or IV). There is a combined reported experience in 217 rheumatoid arthritis patients with liver biopsies both before and during treatment (after a cumulative dose of at least 1500 mg) and in 714 patients with a biopsy only during treatment. There are 64 (7%) cases of fibrosis and 1 (0.1%) case of cirrhosis. Of the 64 cases of fibrosis, 60 were deemed mild. The reticulin stain is more sensitive for early fibrosis and its use may increase these figures. It is unknown whether even longer use will increase these risks. Immune Methotrexate should be used with extreme caution in the presence of active infection, and is contraindicated in patients with overt or laboratory evidence of immunodeficiency syndromes (see CONTRAINDICATIONS). Immunization may be ineffective when given during methotrexate therapy. Immunization with live virus vaccines is generally not recommended. Hypogammaglobulinemia has been reported rarely. Neurologic There have been reports of leukoencephalopathy following intravenous administration of methotrexate to patients who have had craniospinal irradiation. Serious neurotoxicity, frequently manifested as generalized or focal seizures, has been reported with unexpectedly increased frequency among pediatric patients with acute lymphoblastic leukemia who were treated with intravenous methotrexate (1 g/m2). Symptomatic patients were commonly noted to have leukoencephalopathy and/or microangiopathic calcifications on diagnostic imaging studies. Chronic leukoencephalopathy has also been reported in patients with osteosarcoma who received repeated doses of high-dose methotrexate with leucovorin rescue even without cranial irradiation. There are also reports of leukoencephalopathy in patients who received low oral doses (4-8 mg/week) of methotrexate therapy for rheumatoid arthritis or psoriatic arthritis. Discontinuation of methotrexate does not always result in complete recovery. A transient acute neurologic syndrome has been observed in patients treated with high dosage regimens. Manifestations of this neurologic disorder may include behavioural abnormalities, focal sensorimotor signs, including transient blindness and abnormal reflexes. The exact cause is unknown. After the intrathecal use of methotrexate, the central nervous system toxicity which may occur can be classified as follows: chemical arachnoiditis manifested by such symptoms as headache, back pain, nuchal rigidity, and fever; paresis, usually transient, manifested by paraplegia associated with involvement with one or more spinal nerve roots; leucoencephalopathy manifested by confusion, irritability, somnolence, ataxia, dementia, and occasionally major convulsions. Intravenous administration of methotrexate may also result in acute encephalitis and acute encephalopathy with fatal outcome. Cases of severe neurological adverse reactions that ranged from headache to paralysis, coma and stroke-like episodes have been reported mostly in juveniles and adolescents given methotrexate in combination with intravenous cytarabine. Renal Methotrexate is contraindicated in patients with severe renal impairment including end stage renal disease with and without dialysis (see CONTRAINDICATIONS and DOSAGE AND ADMINISTRATION). Methotrexate therapy in patients with mild and moderate renal impairment should be undertaken with extreme caution, and at reduced dosages, because renal dysfunction will prolong methotrexate elimination. Methotrexate may cause renal damage that may lead to acute renal failure. High doses of methotrexate used in the treatment of osteosarcoma may cause renal damage leading to acute renal failure. Nephrotoxicity is due primarily to the precipitation of methotrexate and 7-hydroxymethotrexate in the renal tubules. Close attention to renal function including adequate hydration, urine alkalinization and measurement of serum methotrexate and creatinine levels are essential for safe administration. Nephritis has been reported on co-administration with nitrous oxide anesthesia in rheumatoid arthritis patients (see CONTRAINDICATIONS and DRUG INTERACTIONS: Drug-Drug Interactions). Respiratory Methotrexate-induced lung disease, including acute or chronic interstitial pneumonitis, is a potentially dangerous lesion which may occur at any time during therapy and has been reported at low doses. It is not always fully reversible and fatalities have been reported. Cases of pleural effusion with or without interstitial pneumonitis have also been reported at any time during therapy at low doses. Pulmonary symptoms (especially a dry non-productive cough) or a non-specific pneumonitis occurring during methotrexate therapy may be indicative of a potentially dangerous lesion and require interruption of treatment and careful investigation. Although clinically variable, the typical patient with methotrexate-induced lung disease presents with fever, cough, dyspnea, hypoxemia, and an infiltrate on chest X-ray; infection (including pneumonia) needs to be excluded. This lesion can occur at all dosages. Potentially fatal opportunistic infections, especially Pneumocystis carinii pneumonia, may occur with methotrexate therapy. When a patient presents with pulmonary symptoms, the possibility of Pneumocystis carinii should be considered. Pulmonary alveolar haemorrhage has been reported with methotrexate. This event may also be associated with vasculitis and other comorbidities. Prompt investigations should be considered when pulmonary alveolar haemorrhage is suspected to confirm the diagnosis. Sexual Function/Reproduction Methotrexate causes embryotoxicity, abortion, and fetal defects in humans. It has also been reported to cause impairment of fertility, oligospermia and menstrual dysfunction in humans, during and for a short period after cessation of therapy (see TOXICOLOGY). The risk of effects on reproduction should be discussed with both male and female patients taking methotrexate. (See TOXICOLOGY). Skin Severe, occasionally fatal, dermatologic reactions, including toxic epidermal necrolysis (Lyell's Syndrome), Stevens-Johnson syndrome, exfoliative dermatitis, skin necrosis, and erythema multiforme, have been reported in children and adults, within days of oral, intramuscular or intravenous methotrexate administration. Reactions were noted after single or multiple, low, intermediate or high doses of methotrexate in patients with neoplastic diseases, rheumatoid arthritis or psoriasis. Recovery has been reported with discontinuation of therapy. Lesions of psoriasis may be aggravated by concomitant exposure to ultraviolet radiation. Radiation dermatitis and sunburn may be "recalled" by the use of methotrexate. Special Populations Pregnant Women: Methotrexate is contraindicated in pregnant patients with psoriasis or rheumatoid arthritis (see CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS: Serious Warnings and Precautions) and should be used in the treatment of neoplastic diseases only when the potential benefit outweighs the risk to the fetus. Methotrexate has been reported to cause impairment of fertility, oligospermia and menstrual dysfunction in humans, during and for a short period after cessation of therapy. Methotrexate can cause fetal death, embryotoxicity, abortion, or teratogenic effects when administered to a pregnant woman. Methotrexate is contraindicated in women of childbearing potential until pregnancy is excluded and should be fully counselled on the serious risk to the fetus should they become pregnant while undergoing treatment (see CONTRAINDICATIONS). Pregnancy should be avoided if either partner is receiving methotrexate. The optimal time interval between the cessation of methotrexate treatment of either partner and pregnancy has not been clearly established. Published literature recommendations for time intervals vary from 3 months to one year. The risk of effects on reproduction should be discussed with both male and female patients taking methotrexate . Nursing Women: Methotrexate is contraindicated in nursing mothers because of the potential for serious adverse reactions from methotrexate in breast-fed infants. Pediatrics (<18 years of age): Safety and effectiveness in pediatric patients have not been established, other than in cancer chemotherapy. Overdose by intravenous miscalculation of dosage (particularly in juveniles) have occurred. Special attention must be given to dose calculation. Geriatrics (>65 years of age): The clinical pharmacology of methotrexate has not been well studied in older individuals. Due to diminished hepatic and renal function, as well as decreased folate stores in this population, relatively low doses should be considered. Fatal toxicities related to inadvertent daily rather than weekly dosing have been reported, particularly in elderly patients. Elderly patients should be closely monitored for early signs of hepatic, bone marrow and renal toxicity. Renal Impairment: Methotrexate is contraindicated in patients with severe renal impairment (see CONTRAINDICATIONS and DOSAGE AND ADMINISTRATION). Monitoring and Laboratory Tests General: Patients undergoing methotrexate therapy should be informed of the early signs and symptoms of toxicity and closely monitored so that toxic effects are detected promptly. Serum methotrexate level monitoring can significantly reduce toxicity and mortality by allowing the adjustment of methotrexate dosing and the implementation of appropriate rescue measures. Patients subject to the following conditions are predisposed to developing elevated or prolonged methotrexate levels and benefit from routine monitoring of levels: e.g., pleural effusion, ascites, gastrointestinal tract obstruction, previous cisplatin therapy, dehydration, aciduria, and impaired renal function. Some patients may have delayed methotrexate clearance in the absence of these features. It is important that patients be identified within 48 hours since methotrexate toxicity may not be reversible if adequate leucovorin rescue is delayed for more than 42 to 48 hours. Monitoring of methotrexate concentrations should include determination of a methotrexate level at 24, 48, or 72 hours, and assessment of the rate of decline in methotrexate concentrations (to determine how long to continue leucovorin rescue). Baseline assessment should include a complete blood count with differential and platelet counts, hepatic enzymes, renal function tests, and a chest X-ray. During initial or changing doses, or during periods of increased risk of elevated methotrexate blood levels (e.g., dehydration), more frequent monitoring may also be indicated. During therapy of rheumatoid arthritis and psoriasis, monitor: - Hematologic: Patients should have their blood tests checked at least monthly. - Hepatic: Liver biopsies prior to methotrexate therapy are not indicated routinely. Liver function tests should be determined prior to the initiation of therapy with methotrexate and they should be monitored every 1 to 2 months. A relationship between abnormal liver function tests and fibrosis or cirrhosis of the liver has not been established. Transient liver function test abnormalities are observed frequently after methotrexate administration and are usually not cause for modification of methotrexate therapy. Persistent liver function test abnormalities just prior to dosing and/or depression of serum albumin may be indicators of serious liver toxicity and require evaluation. - Renal: Renal function should be monitored every 1 to 2 months. - Respiratory: Pulmonary function tests may be useful if methotrexate-induced lung disease (e.g. interstitial pneumonitis) is suspected, especially if baseline measurements are available. During therapy of neoplastic disease: More frequent monitoring is usually indicated during antineoplastic therapy for hematologic, hepatic, renal and respiratory. Excipient information Abitrexate Teva 2 ml, 4 ml and 8 ml vials contain less than 1 mmol sodium (23 mg) per vial, that is to say essentially 'sodium free'. Abitrexate Teva 20 ml contains approximately 38.6 mg sodium per vial, equivalent to 1.93% of the WHO recommended maximum daily intake of 2 g sodium for an adult. Abitrexate Teva 40 ml contains approximately 77.2 mg sodium per vial, equivalent to 3.86% of the WHO recommended maximum daily intake of 2 g sodium for an adult. ADVERSE REACTIONS Adverse Drug Reaction Overview In general, the incidence and severity of acute side effects are related to dose, frequency of administration, and the duration of the exposure to significant blood levels of methotrexate to the target organs. The most serious reactions are discussed under WARNINGS AND PRECAUTIONS section. The most frequently reported adverse reactions include ulcerative stomatitis, leucopenia, nausea, and abdominal distress. Other frequently reported adverse effects are malaise, undue fatigue, chills and fever, dizziness and decreased resistance to infection. Ulcerations of the oral mucosa are usually the earliest signs of toxicity. Adverse Drug Reactions by Organ System Blood and lymphatic Leucopenia, anemia, aplastic anemia, thrombopenia, system disorders: pancytopenia, agranulocytosis, lymphadenopathy and lymphoproliferative disorders (including reversible), neutropenia and eosinophilia have also been observed. Cardiac disorders: Pericarditis and pericardial effusion (damage to heart, rarely). Eye disorders: Conjunctivitis, blurred vision, serious visual changes of unknown etiology, and transient blindness/vision loss. Gastrointestinal Gingivitis, stomatitis, enteritis, anorexia, nausea, disorders: vomiting, diarrhea, hematemesis, melena, gastrointestinal ulceration and bleeding, pancreatitis, intestinal perforation, non-infectious peritonitis, glossitis. General disorders and Anaphylactoid reactions, vasculitis, fever, administration site conjunctivitis, infection, sepsis, nodulosis, conditions: hypogammaglobulinaemia, and sudden death. Hepatobiliary disorders: Hepatotoxicity, acute hepatitis, chronic fibrosis and cirrhosis, decrease in serum albumin, liver enzyme elevations, hepatic failure. Infection: Other reported infections included nocardiosis, histoplasmosis, cryptococcosis, and disseminated H. simplex, cytomegalovirus infection, including cytomegaloviral pneumonia. Metabolism and Diabetes mellitus. nutrition disorders: Musculoskeletal, Stress fractures, soft tissue necrosis, osteonecrosis, connective tissue arthralgia, myalgia and osteoporosis. and bone disorders: Neoplasms benign, malignant and unspecified Tumour lysis syndrome. Malignant lymphomas, (including cysts and polyps): which may regress following withdrawal of methotrexate, may occur in patients receiving low- dose methotrexate, and thus may not require cytotoxic treatment. Discontinue methotrexate first and, if the lymphoma does not regress, appropriate treatment should be instituted. Nervous system: Cerebrospinal fluid pressure increased, neurotoxicity, arachnoiditis, paresthesia, headache, dizziness, drowsiness, speech impediment including dysarthria and aphasia; hemiparesis, paresis and convulsions. Following low doses, there have been occasional reports of transient subtle cognitive dysfunction, mood alteration, or unusual cranial sensations, leukoencephalopathy, or encephalopathy. Renal and urinary Renal failure, severe nephropathy or renal failure, disorders: azotemia, dysuria, cystitis, hematuria, urogenital dysfunction. Proteinuria has also been observed. Reproductive system Defective oogenesis or spermatogenesis, transient and breast disorders: oligospermia, menstrual dysfunction, vaginal discharge and gynecomastia; infertility, abortion, fetal defects, loss of libido/impotence. Respiratory, thoracic Pneumonia, interstitial alveolitis/pneumonitis often and mediastinal disorders: associated with eosinophilia, pulmonary fibrosis, Pneumocystis carinii pneumonia, pleural effusion. Dyspnea, chest pain, hypoxia, respiratory fibrosis, pharyngitis, and chronic interstitial obstructive pulmonary disease, alveolitis and pulmonary alveolar haemorrhage have occasionally occurred. Skin disorders: Erythema, pruritus, photosensitisation, petechiae, loss of hair, skin necrosis, exfoliative dermatitis, painful erosion of psoriatic plaques, herpes zoster, vasculitis, urticaria, pigmentary changes, acne, ecchymosis, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome), furunculosis and telangiectasia. Drug reaction with eosinophilia and systemic symptoms Vascular disorders: Hypotension, and thromboembolic events (including arterial thrombosis, cerebral thrombosis, deep vein thrombosis, retinal vein thrombosis, thrombophlebitis, and pulmonary embolus), vasculitis Adverse Reactions Reported in Rheumatoid Arthritis: • Alopecia (common) • Diarrhea (common) • Dizziness (common) • Elevated liver enzymes (very common) • Leucopenia (common) • Nausea/vomiting (very common) • Pancytopenia (common) • Rash/pruritus/dermatitis (common) • Stomatitis (common) • Thrombocytopenia (common) Adverse Reactions in Psoriasis: The adverse reaction rates reported are very similar to those in the rheumatoid arthritis studies. Rarely, painful psoriatic plaque erosions may appear. Abnormal Hematologic and Clinical Chemistry Findings See WARNINGS AND PRECAUTIONS: Monitoring and Laboratory Tests section. Post-Market Adverse Drug Reactions Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse events have also been reported during post-marketing experience with methotrexate: System Organ Class Adverse Reaction Infections (including fatal sepsis); Pneumonia; Pneumocystis carinii pneumonia; Nocardiosis; Histoplasmosis; Cryptococcosis; Herpes zoster; Infections and Infestations H. simplex hepatitis; Disseminated H. simplex; Cytomegalovirus infection (including cytomegaloviral pneumonia); Reactivation of hepatitis B infection; Worsening of hepatitis C infection Agranulocytosis; Pancytopenia; Leukopenia; Neutropenia; Lymphadenopathy and lymphoproliferative Blood and Lymphatic System Disorders disorders (including reversible); Eosinophilia; Anemia megaloblastic; Renal vein thrombosis; Lymphoma; Aplastic anemia; Hypogammaglobulinemia CSF pressure increased; Neurotoxicity; Arachnoiditis; Nervous System Disorders Paraplegia; Stupor; Ataxia; Dementia; Dizziness; Paresthesia Chronic interstitial pulmonary disease; Respiratory, Thoracic and Mediastinal Disorders Alveolitis; Dyspnea; Chest pain; Hypoxia; Cough; Plural effusion Intestinal perforation; Noninfectious peritonitis; Gastrointestinal Disorders Glossitis; Nausea; Pancreatitis Hepatobiliary Disorders Hepatic failure Drug reaction with eosinophilia and systemic symptoms; Skin and Subcutaneous Tissue Disorders Dermatitis; Petechiae Musculoskeletal, Connective Tissue and Bone Disorders Osteonecrosis Renal and Urinary Disorders Proteinuria Pregnancy, Puerperium and Perinatal Conditions Fetal death, Abortion Reproductive System and Breast Disorders Urogenital dysfunction General Disorders and Administration Site Conditions Pyrexia; Chills; Malaise; Fatigue; Anaphylactic reactions Endocrine Disorders Diabetes Ophthalmologic Disorders Transient blindness/vision loss Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il. DRUG INTERACTIONS Serious Drug Interactions The use of nitrous oxide anesthesia with methotrexate is contraindicated (see CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS - Renal and DRUG INTERACTIONS - Drug-Drug Interactions). Overview Methotrexate competes with reduced folates for active transport across cell membranes by means of a single carrier-mediated active transport process. Impaired renal function, as well as concurrent use of drugs such as weak organic acids that undergo tubular secretion, can markedly increase methotrexate serum levels. Laboratory studies demonstrate that methotrexate may be displaced from plasma albumin by various compounds including sulfonamides, salicylates, tetracyclines, chloramphenicol and phenytoin. Drug-Drug Interactions Non-steroidal Anti-inflammatory Drugs (NSAIDs) NSAIDs should not be administered prior to or concomitantly with high doses of methotrexate. Concomitant administration of NSAIDs with high-dose methotrexate therapy has been reported to elevate and prolong serum methotrexate levels, resulting in deaths from severe hematologic (including bone marrow suppression and aplastic anemia) and gastrointestinal toxicity. These drugs have been reported to reduce the tubular secretion of methotrexate, in an animal model, and may enhance its toxicity by increasing methotrexate levels. Caution should be used when NSAIDs and salicylates are administered concomitantly with lower doses of methotrexate . In treating rheumatoid arthritis with methotrexate, the possibility of increased toxicity with concomitant use of NSAIDs including salicylates has not been fully explored. Despite the potential interactions, studies of methotrexate in patients with rheumatoid arthritis have usually included concurrent use of constant dosage regimens of NSAIDs without apparent problems. It should be appreciated however, that the doses used in rheumatoid arthritis (7.5 to 15 mg/week) are somewhat lower than those used in psoriasis and that larger doses could lead to toxicity. Disease Modifying Antirheumatic drugs (DMARDs) Combined use of methotrexate with gold, penicillamine, hydroxychloroquine, or sulfasalazine has not been studied and may increase the incidence of adverse effects. Amiodarone Amiodarone administration to patients receiving methotrexate treatment for psoriasis has induced ulcerated skin lesions. L-asparaginase The administration of L-asparaginase has been reported to antagonize the effect of methotrexate. Diuretics Bone marrow suppression and decreased folate levels have been described in the concomitant administration of triamterene and methotrexate. Leflunomide Methotrexate in combination with leflunomide may increase the risk of pancytopenia. Drugs Highly Bound to Plasma Proteins Methotrexate is partially bound to serum albumin, and toxicity may be increased because of displacement by other highly bound drugs, such as sulfonylureas, aminobenzoic acid, salicylates, phenylbutazone, phenytoin, sulfonamides, some antibiotics such as penicillins, tetracycline, pristinamycin, probenecid, and chloramphenicol. Packed Red Blood Cells Care should be exercised whenever packed red blood cells and methotrexate are given concurrently. Patients receiving 24-hr methotrexate infusion and subsequent transfusions have showed enhanced toxicity probably resulting from prolonged high serum-methotrexate concentrations. Probenecid Renal tubular transport is also diminished by probenecid; use of methotrexate with this drug should be carefully monitored. Proton Pump Inhibitors Use caution when administering high-dose methotrexate to patients receiving proton pump inhibitor (PPI) therapy. Concomitant use of PPIs and high-dose methotrexate should be avoided especially in patients with renal impairment. Case reports and published population pharmacokinetic studies suggest that concomitant use of some PPIs, such as omeprazole, esomeprazole, and pantoprazole, with methotrexate (primarily at high dose), may elevate and prolong serum levels of methotrexate and/or its metabolite hydromethotrexate, possibly leading to methotrexate toxicities. In two of these cases, delayed methotrexate elimination was observed when high-dose methotrexate was co-administered with PPIs, but was not observed when methotrexate was co-administered with ranitidine. However, no formal drug interaction studies of methotrexate with ranitidine have been conducted. Psoralen Plus Ultraviolet Light (PUVA) Therapy Skin cancer has been reported in patients with psoriasis or mycosis fungoides (a cutaneous T-cell lymphoma) receiving a concomitant treatment with methotrexate plus PUVA therapy. Nephrotoxic Drugs In the treatment of patients with osteosarcoma, caution must be exercised if high-dose methotrexate is administered in combination with a potentially nephrotoxic chemotherapeutic agent (e.g., cisplatin). Methotrexate clearance is decreased by cisplatinum. Although not documented, other nephrotoxic drugs such as aminoglycosides, Amphotericin B and Cyclosporin could theoretically increase methotrexate toxicity by decreasing its elimination. Nitrous Oxide The use of nitrous oxide anesthesia potentiates the effect of methotrexate on folate metabolism, yielding increased toxicity such as severe, unpredictable myelosuppression, stomatitis, neurotoxicity (with intrathecal administration of methotrexate) and nephritis (see CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS: Renal). In case of accidental co-administration, this effect can be reduced by the use of leucovorin rescue. Penicillins and Sulfonamides Penicillins and sulfonamides may reduce the renal clearance of methotrexate; hematologic and gastrointestinal toxicity have been observed in combination with methotrexate. Use of methotrexate with penicillins should be carefully monitored. Ciprofloxacin Renal tubular transport is diminished by ciprofloxacin; use of methotrexate with this drug should be carefully monitored. Oral Antibiotics Oral antibiotics such as tetracycline, chloramphenicol, and nonabsorbable broad spectrum antibiotics, may decrease intestinal absorption of methotrexate or interfere with the enterohepatic circulation by inhibiting bowel flora and suppressing metabolism of the drug by bacteria. For example: Neomycin, Polymyxin B, Nystatin and Vancomycin decrease methotrexate absorption, whereas Kanamycin increases methotrexate absorption. Trimethoprim/sulfamethoxazole has been reported rarely to increase bone marrow suppression in patients receiving methotrexate, probably by decreased tubular secretion and/or an additive antifolate effect. Concurrent use of the anti-protozoal pyrimethamine may increase the toxic effects of methotrexate because of an additive antifolate effect. Theophylline Methotrexate may decrease the clearance of theophylline; theophylline levels should be monitored when used concurrently with methotrexate. Mercaptopurine Methotrexate increases the plasma levels of mercaptopurine. Combination of methotrexate and mercaptopurine may therefore require dose adjustment. Vitamins Vitamin preparations containing folic acid or its derivatives may decrease responses to methotrexate. Preliminary animal and human studies have shown that small quantities of intravenously administered leucovorin enter the cerebrospinal fluid primarily as 5-methyl tetrahydrofolate and, in humans, remain 1 to 3 orders of magnitude lower than the usual methotrexate concentrations following intrathecal administration. However, high doses of leucovorin may reduce the efficacy of intrathecally administered methotrexate. In patients with rheumatoid arthritis or psoriasis, folic acid or folinic acid may reduce methotrexate toxicities such as gastrointestinal symptoms, stomatitis, alopecia, and elevated liver enzymes. Before taking a folate supplement, it is advisable to check B12 levels, particularly in adults over the age of 50, since folate administration can mask symptoms of B12 deficiency. Folate deficiency states may increase methotrexate toxicity. Radiotherapy Methotrexate given concomitantly with radiotherapy may increase the risk of soft tissue necrosis and osteonecrosis. Hepatoxins The potential for increased hepatotoxicity when methotrexate is administered with other hepatotoxic agents has not been evaluated. However, hepatotoxicity has been reported in such cases. Therefore, patients receiving concomitant therapy with methotrexate and other potential hepatotoxic agents (e.g., leflunomide, azathioprine, sulfasalazine, retinoids) should be closely monitored for possible increased risk of hepatotoxicity. Cytarabine and other cytotoxic agents Methotrexate given concomitantly with I.V. cytarabine may increase the risk of severe neurologic adverse events such as headache, paralysis, coma and stroke-like episodes (see WARNINGS AND PRECAUTIONS: Neurologic). Combined use of methotrexate with other cytotoxic agents has not been studied and may increase the incidence of adverse effects. Drug-Herb Interactions The effects of herbal products on the pharmacokinetics of methotrexate have not been studied. Drug-Laboratory Interactions Interactions with laboratory tests have not been established. Drug-Lifestyle Interactions Use of alcohol with methotrexate is contraindicated (see CONTRAINDICATIONS). The effects of smoking, on the pharmacokinetics of methotrexate have not been specifically studied. Some of the effects (e.g., dizziness and fatigue) may have an influence on the ability to drive or operate machinery. DOSAGE AND ADMINISTRATION

שימוש לפי פנקס קופ''ח כללית 1994
Leukemias, non-hodgkin's lymphomas, breast, head and lung carcinoma, choriocarcinoma, osteogenic sarcoma. Severe psoriasis, rheumatoid arthritis unresponsive to conventional therapy, mycosis fungoides
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
