Quest for the right Drug
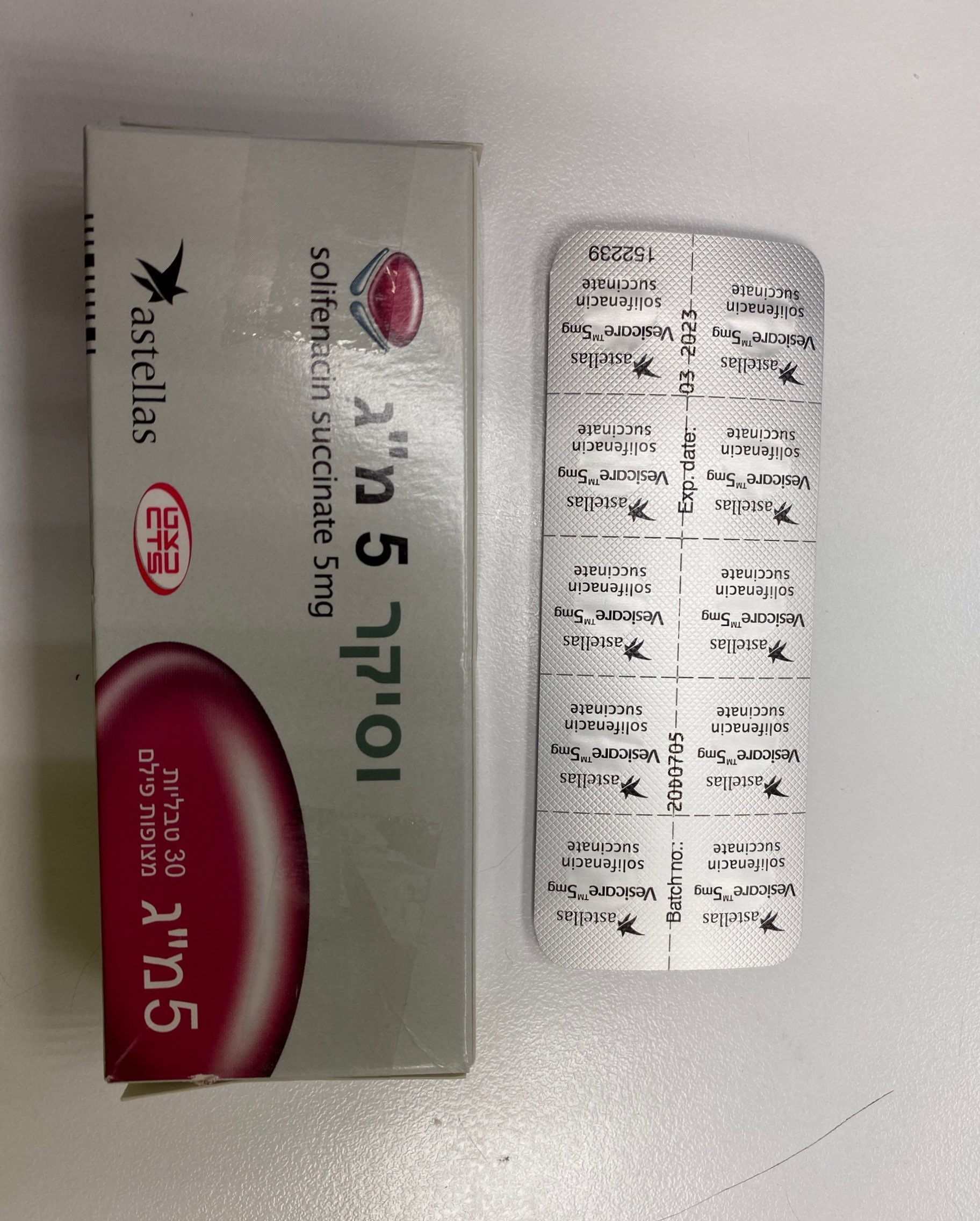
וסיקר 5 מ"ג VESICARE 5 MG (SOLIFENACIN SUCCINATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מצופות פילם : FILM COATED TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Due to the pharmacological effect of solifenacin, Vesicare may cause anticholinergic undesirable effects of (in general) mild or moderate severity. The frequency of anticholinergic undesirable effects is dose related. The most commonly reported adverse reaction with Vesicare was dry mouth. It occurred in 11% of patients treated with 5 mg once daily, in 22% of patients treated with 10 mg once daily and in 4% of placebo-treated patients. The severity of dry mouth was generally mild and did only occasionally lead to discontinuation of treatment. In general, medicinal product compliance was very high (approximately 99%) and approximately 90% of the patients treated with Vesicare completed the full study period of 12 weeks treatment. Tabulated list of adverse reactions -5- MedDRA Very Common Uncommon Rare Very rare Not known system organ commo ≥1/100, ≥1/1000, ≥ 1/10000, <1/10,000, (cannot be class n ≥1/10 <1/10 <1/100 <1/1000 estimated form the available data) Infections and Urinary tract infestations infection Cystitis Immune Anaphylactic system reaction* disorders Metabolism Decreased and nutrition appetite* disorders Hyperkalaemia * Psychiatric Hallucinations Delirium* disorders * Confusional state* Nervous Somnolence Dizziness* system Dysgeusia , disorders Headache * Eye disorders Blurred Dry eyes Glaucoma* vision Cardiac Torsade de disorders Pointes* Electrocardiogr am QT prolonged* Atrial fibrillation* Palpitation* Tachycardia* Respiratory, Nasal Dysphonia* thoracic and dryness mediastinal disorders Gastrointestina Dry Constipatio Gastro- Colonic Ileus* l disorders mouth n oesophagea obstructio Abdominal Nausea l reflux n discomfort* Dyspepsia diseases Faecal Abdominal Dry throat impaction pain Vomiting* Hepatobiliary Liver disorder* disorders Liver function test abnormal* -6- Skin and Dry skin Pruritus*, Erythema Exfoliative subcutaneous Rash*, multiforme*, dermatitis* tissue Urticaria* disorders Angioedema* Musculoskeleta Muscular l and weakness* connective tissue disorders Renal and Difficulty in Urinary Renal urinary micturition retention impairment* disorders General Fatigue disorders and Peripheral administration oedema site conditions * observed post-marketing Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the national regulation by using an online form https://sideeffects.health.gov.il/

מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| לטיפול בשלפחות שתן פעילה ביתר |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/2009
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
