Quest for the right Drug
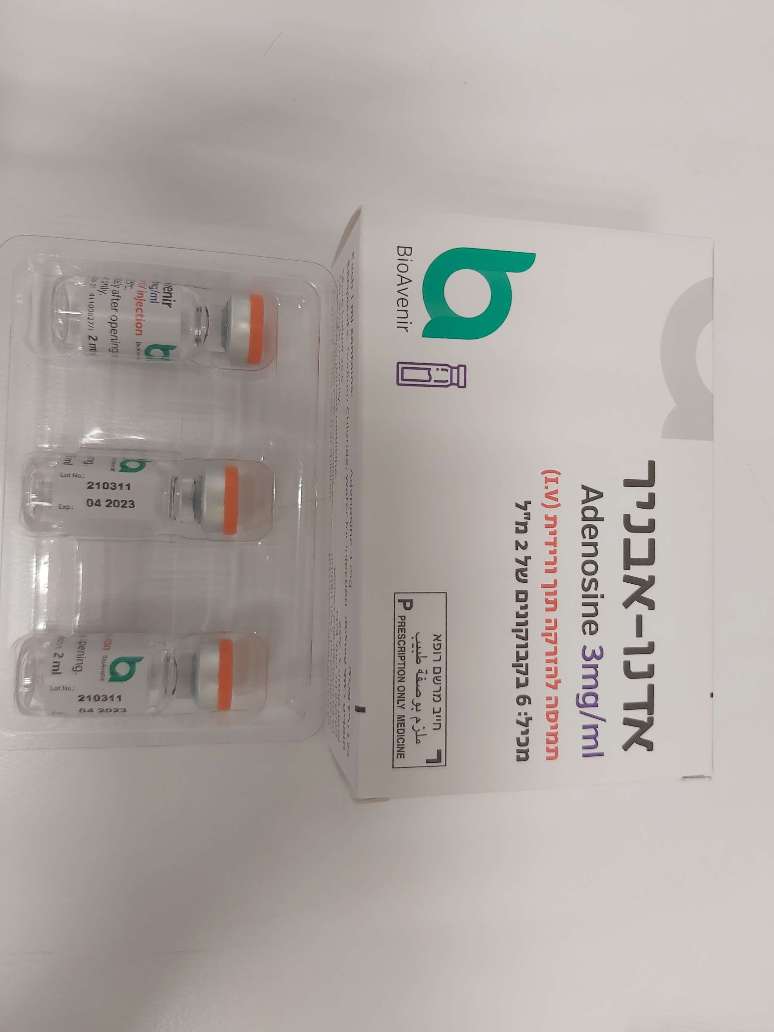
אדנו-אבניר ADENO-AVENIR (ADENOSINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects These side effects are generally mild, of short duration (usually less than 1 minute) and well tolerated by the patient. However severe reactions can occur. Methylxanthines, such as IV aminophylline or theophylline have been used to terminate persistent side effects (50 – 125 mg by slow intravenous injection). Adverse events are ranked under the heading of the frequency: Very common (> 1/10), common (> 1/100 to < 1/10), uncommon (> 1/1000 to < 1/100), rare (> 1/10000 to < 1/1000), very rare (< 1/10000), not known (cannot be estimated from available data). Immune system disorders: Not known: anaphylactic reaction (including angioedema and skin reactions such as urticaria and rash). Cardiac disorders: Very common: bradycardia, sinus pause, skipped beats, atrial extrasystoles, Atrio- Ventricular block, ventricular excitability disorders such as ventricular extrasystoles, non-sustained ventricular tachycardia Uncommon: sinus tachycardia, palpitations Very rare: atrial fibrillation, severe bradycardia not corrected by atropine and possibly requiring temporary pacing, ventricular excitability disorders, including ventricular fibrillation and torsade de pointes (see section 4.4) Not known: asystole/cardiac arrest, sometimes fatal especially in patients with underlying ischemic heart disease/cardiac disorder (see section 4.4). Arteriospasm coronary which may lead to myocardial infarction. Vascular disorders: Very common: flushing Not known: hypotension (sometimes severe) (see section 4.4) Nervous system disorders: Common: headache, dizziness, light-headedness, paraesthesia Uncommon: head pressure Very rare: transient and spontaneously rapidly reversible worsening of intracranial hypertension Not known: loss of consciousness/syncope, convulsions, especially in predisposed patients (see section 4.4) Eye disorders: Uncommon: blurred vision Respiratory, thoracic and mediastinal disorders: Very common: dyspnea (or the urge to take a deep breath) Uncommon: hyperventilation Very rare: bronchospasm (see section 4.4) Not known: respiratory failure (see section 4.4), apnoea/respiratory arrest Cases of respiratory failure, bronchospasm, apnoea, and respiratory arrest with fatal outcome have been reported. Gastrointestinal disorders: Common: nausea Uncommon: metallic taste Not known: vomiting Psychiatric disorders: Common: nervousness General disorders and administration site conditions: Very common: chest pain or pressure, feeling of thoracic constriction/oppression Uncommon: sweating, discomfort in the leg, arm or back, feeling of general discomfort, weakness/pain Very rare: injection site reactions Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2001
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
