Quest for the right Drug
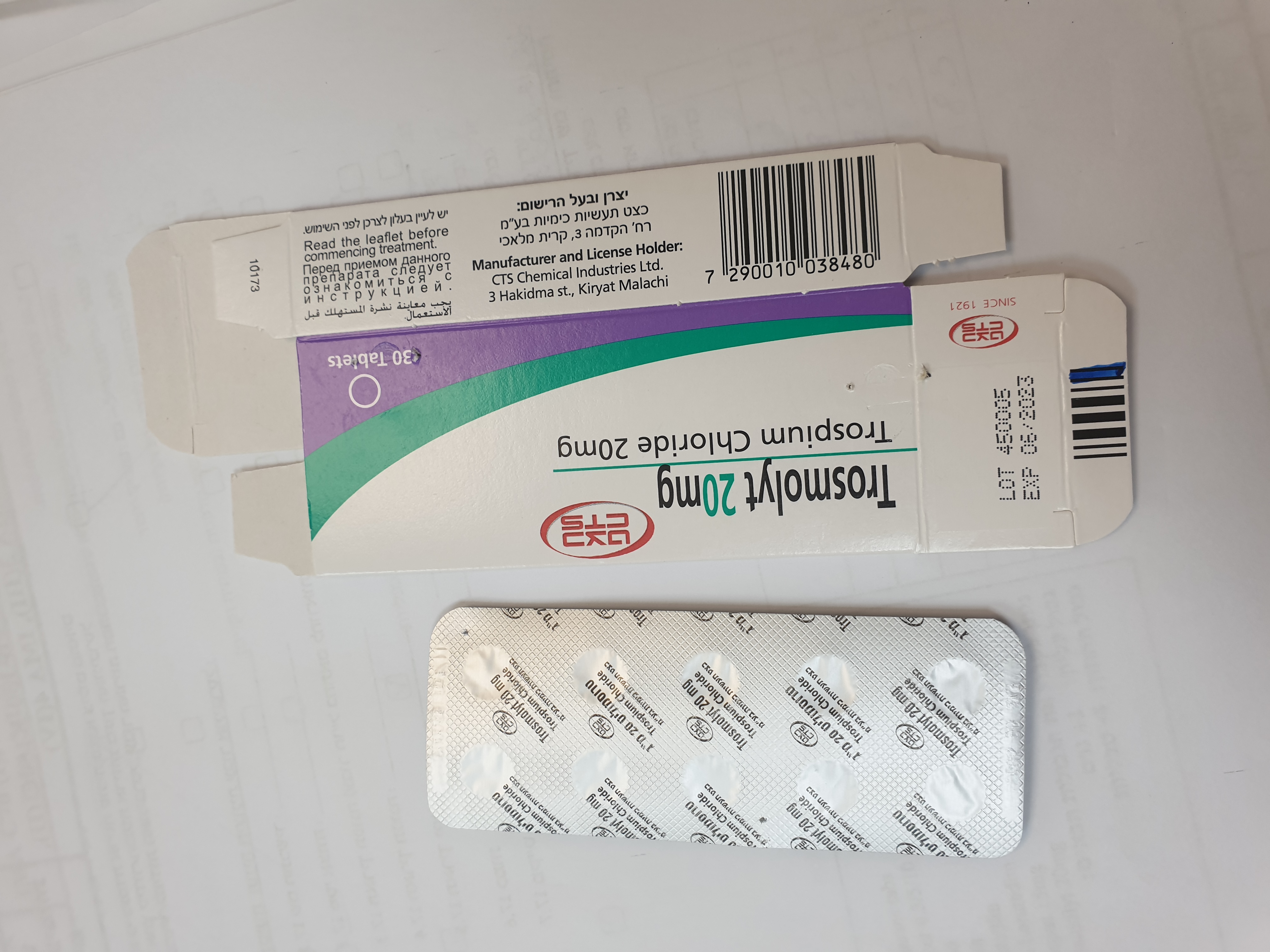
טרוסמוליט 20 מ"ג TROSMOLYT 20 MG (TROSPIUM CHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מצופות פילם : FILM COATED TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Undesirable effects observed with trospium chloride such as dry mouth, dyspepsia and constipation mainly reflect the typical anticholinergic properties of the active ingredient. In Phase- III clinical studies, dry mouth was very common and occurred in approximately 18% of patients treated with trospium chloride and in approximately 6% treated with placebo (total of 1931 patients of which 911 received placebo). Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare (≥1/10,000 to <1/1000) and very rare (<1/10,000). The following table lists possibly related drug reactions reported for patients treated with Trosmolyt 20mg film-coated tablets: Very Common Uncommon Rare Very Rare Not known (cannot be common estimated from the available data) Cardiac disorders Tachycardia Tachyarrhythmia Nervous system Headache Dizziness Hallucination* disorders confusion* agitation* Eye disorders Vision disorders Respiratory, Dyspnoea thoracic and mediastinal disorders Gastrointestinal Dry Dyspepsia Flatulence disorders mouth Constipation Diarrhoea Abdominal pain Nausea Renal and urinary Micturition disorders disorders Urinary retention Skin and Rash Angio-oedema Pruritus subcutaneous Urticaria disorders Stevens-Johnson Syndrome (SJS) / Toxic Epidermal Necrolysis (TEN) Muscoskeletal and Myalgia connective tissue Arthralgia disorders General disorders Chest pain Asthenia and administration site conditions Immune system Anaphylaxis disorders Investigations Mild to moderate increase in serum transaminase levels *These adverse effects occurred mostly in elderly patients and can be facilitated by neurological diseases and/or concomitant intake of other anticholinergic drugs (see section 4.5). Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| לטיפול בשלפחות שתן פעילה ביתר | FESOTERODINE, SOLIFENACIN, TOLTERODINE, TROSPIUM |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
