Quest for the right Drug
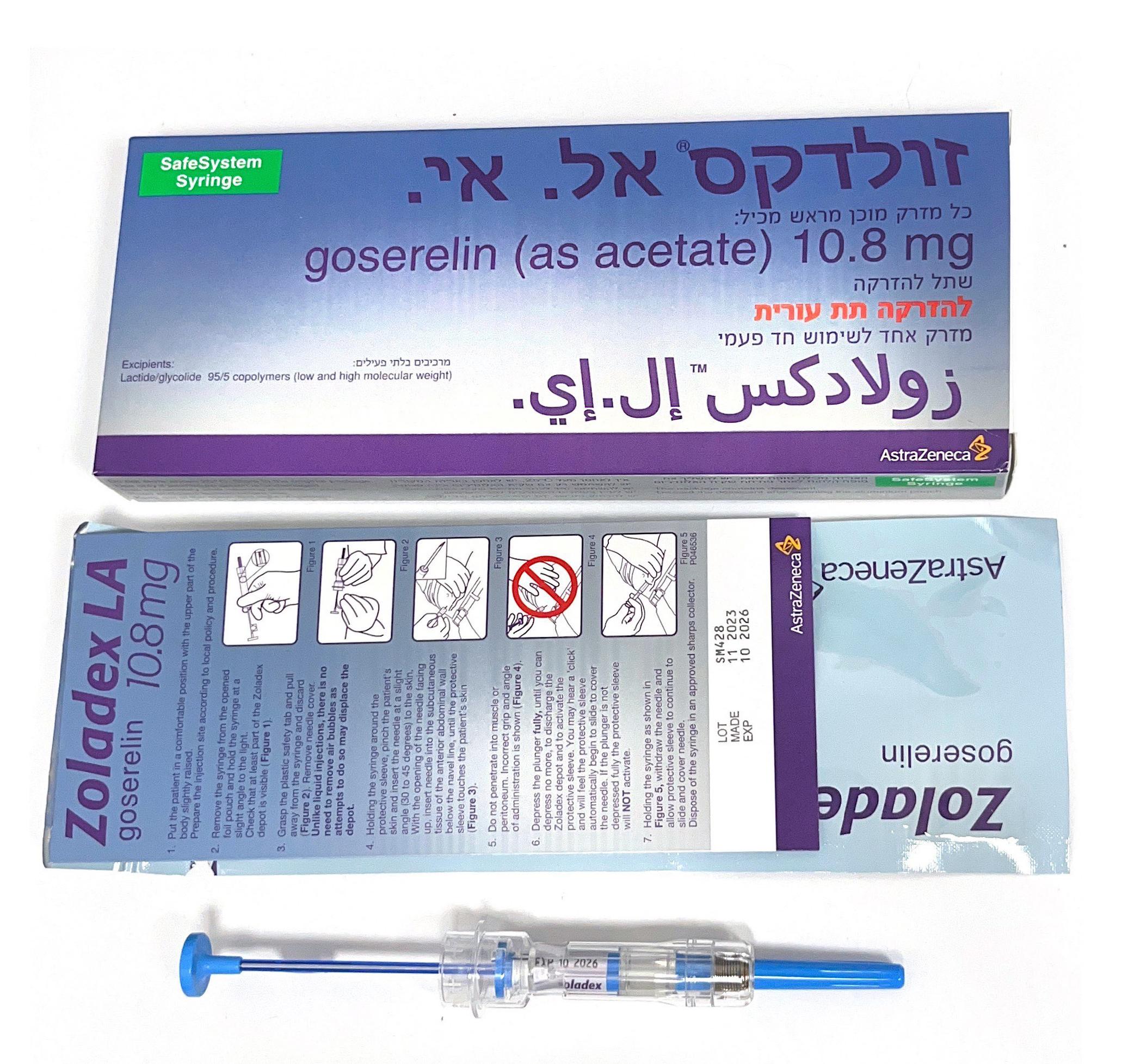
זולדקס אל.אי. ZOLADEX LA (GOSERELIN AS ACETATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
שתל להזרקה : IMPLANT FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapuetic group: Gonadotropin releasing hormone analogues, ATC code: L02AE03. ZOLADEX (D-Ser(But)6Azgly10 LHRH) is a synthetic analogue of naturally occurring LHRH. On chronic administration ZOLADEX LA results in inhibition of pituitary LH secretion leading to a fall in serum testosterone concentrations in males. This effect is reversible on discontinuation of therapy. Initially, ZOLADEX LA like other LHRH agonists, may transiently increases serum testosterone concentrations. In men by around 21 days after the first depot injection, testosterone concentrations have fallen to within the castrate range and remain suppressed with treatment every 12 weeks. In the management of patients with metastatic prostate cancer, ZOLADEX has been shown in comparative clinical trials to give similar survival outcomes to those obtained with surgical castrations. In a combined analysis of 2 randomised controlled trials comparing bicalutamide 150 mg monotherapy versus castration (predominantly in the form of ZOLADEX), there was no significant difference in overall survival between bicalutamide-treated patients and castration-treated patients (hazard ratio = 1.05 [CI 0.81 to 1.36]) with locally advanced prostate cancer. However, equivalence of the two treatments could not be concluded statistically. In comparative trials, ZOLADEX has been shown to improve disease-free survival and overall survival when used as an adjuvant therapy to radiotherapy in patients with high risk localised (T1-T2 and PSA of at least 10 ng/mL or a Gleason score of at least Page 7 of 10 8 7), or locally advanced (T3-T4) prostate cancer. The optimum duration of adjuvant therapy has not been established; a comparative trial has shown that 3 years of adjuvant ZOLADEX gives significant survival improvement compared with radiotherapy alone. Neo-adjuvant ZOLADEX prior to radiotherapy has been shown to improve disease-free survival in patients with high risk localised or locally advanced prostate cancer. After prostatectomy, in patients found to have extra-prostatic tumour spread, adjuvant ZOLADEX may improve disease free survival periods, but there is no significant survival improvement unless patients have evidence of nodal involvement at time of surgery. Patients with pathologically staged locally advanced disease should have additional risk factors such as PSA of at least 10 ng/mL or a Gleason score of at least 7 before adjuvant ZOLADEX should be considered. There is no evidence of improved clinical outcomes with use of neo-adjuvant ZOLADEX before radical prostatectomy.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Administration of ZOLADEX LA every 12 weeks ensures that exposure to goserelin is maintained with no clinically significant accumulation. ZOLADEX is poorly protein bound and has a serum elimination half-life of two to four hours in subjects with normal renal function. The half-life is increased in patients with impaired renal function. For the compound given in a 10.8 mg depot formulation every 12 weeks this change will not lead to any accumulation. Hence, no change in dosing is necessary in these patients. There is no significant change in pharmacokinetics in patients with hepatic failure.

מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| Breast cancer for premenopausal women | אונקולוגיה | GOSERELIN, LEUPRORELIN |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
09/03/1999
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
