Quest for the right Drug
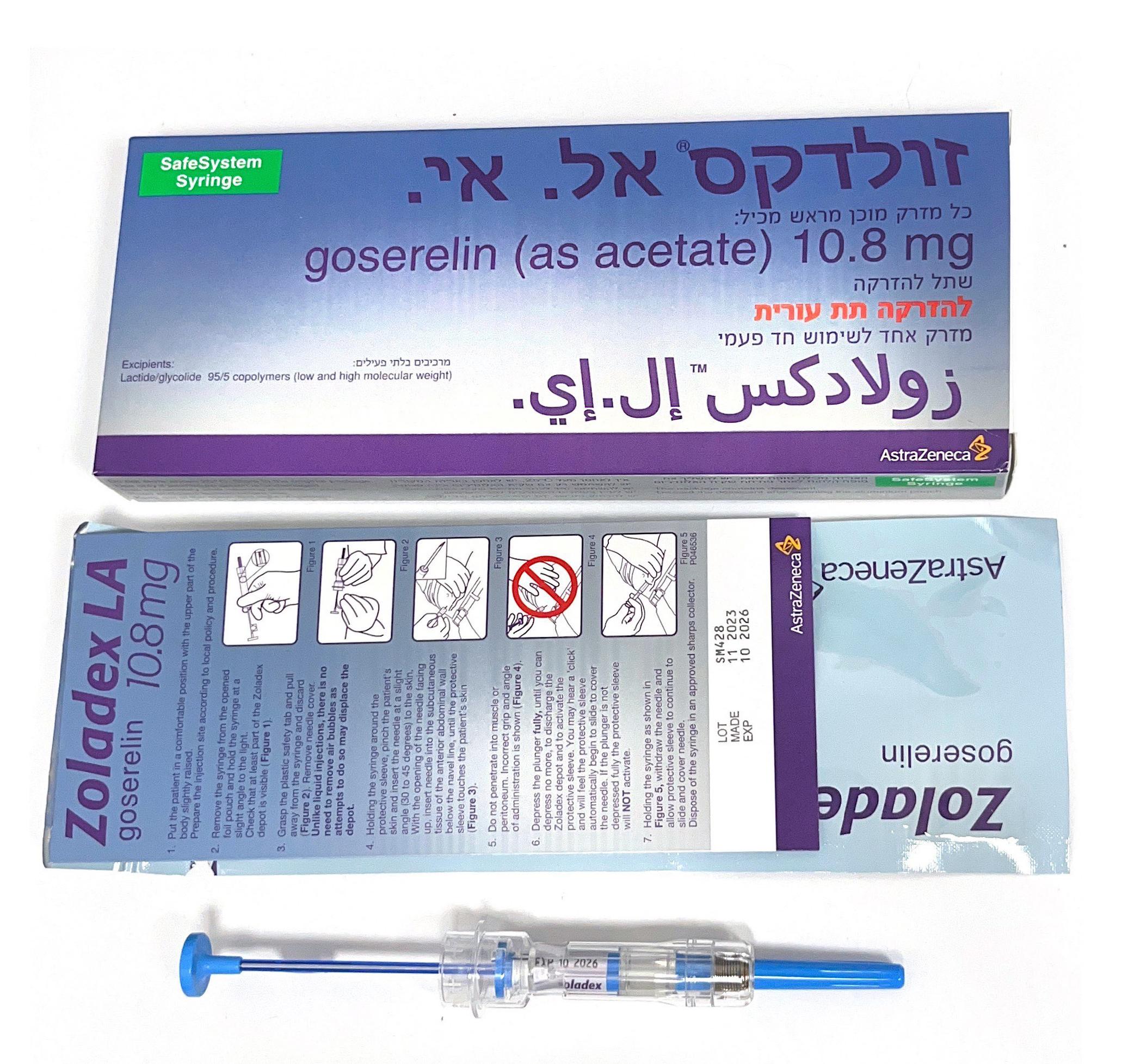
זולדקס אל.אי. ZOLADEX LA (GOSERELIN AS ACETATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
שתל להזרקה : IMPLANT FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use There is no data on removal or dissolution of the implant. There is an increased risk of incident depression (which may be severe) in patients undergoing treatment with GnRH agonists, such as Goserelin. Patients should be informed accordingly and treated as appropriate if symptoms occur. Carefully monitor patients with known depression or history of depression. Androgen deprivation therapy may prolong the QT interval. In patients with a history of or risk factors for QT prolongation and in patients receiving concomitant medicinal products that might prolong the QT interval (see section 4.5) physicians should assess the benefit risk ratio including the potential for Torsade de pointes prior to initiating Zoladex LA. Injection site injury has been reported with ZOLADEX LA, including events of pain, haematoma, haemorrhage and vascular injury. Monitor affected patients for signs or symptoms of abdominal haemorrhage. In very rare cases, administration error resulted in vascular injury and haemorrhagic shock requiring blood transfusions and surgical intervention. Extra care should be taken when administering ZOLADEX LA to patients with a low BMI and/or receiving full anticoagulation medications (see Section 4.2). Treatment with Zoladex LA may lead to positive reactions in anti-doping tests. Page 2 of 10 3 Patients with hypertension should be monitored carefully, as should patients with risk factors for diabetes with treatment initiated, if appropriate, according to national guidelines. ZOLADEX LA is not indicated for use in females, since there is insufficient evidence of reliable suppression of serum oestradiol. For female patients requiring treatment with goserelin, refer to the prescribing information for ZOLADEX 3.6 mg. The use of ZOLADEX LA in patients at particular risk of developing ureteric obstruction or spinal cord compression should be considered carefully and the patients monitored closely during the first month of therapy. If spinal cord compression or renal impairment due to ureteric obstruction are present or develop, specific standard treatment of these complications should be instituted. Consideration should be given to the initial use of an antiandrogen (e.g. cyproterone acetate 300 mg daily for three days before and three weeks after commencement of ZOLADEX) at the start of LHRH analogue therapy since this has been reported to prevent the possible sequelae of the initial rise in serum testosterone. The use of LHRH agonists in men may cause a reduction in bone mineral density. In men, preliminary data suggest the use of a bisphosphonate in combination with a LHRH agonist may reduce mineral loss. Particular caution is necessary in patients with additional risk factors for osteoporosis (e.g. chronic alcohol abusers, smokers, long-term therapy with anticonvulsants or corticosteroids, family history of osteoporosis). Myocardial infarction and cardiac failure were observed in a pharmaco-epidemiology study of LHRH agonists used in the treatment of prostate cancer. The risk appears to be increased when used in combination with anti-androgens. Reduction in glucose tolerance has been observed in males receiving LHRH agonists. This may manifest as diabetes or loss of glycaemic control in patients with pre-existing diabetes mellitus. Thus, monitoring of blood glucose levels should be considered. Page 3 of 10 4 Paediatric population ZOLADEX LA is not indicated for use in children, as safety and efficacy have not been established in this patient group .
Effects on Driving
4.7 Effects on ability to drive and use machines Zoladex LA has no or negligible influence on the ability to drive and use machinery.

מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| Breast cancer for premenopausal women | אונקולוגיה | GOSERELIN, LEUPRORELIN |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
09/03/1999
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
