Quest for the right Drug
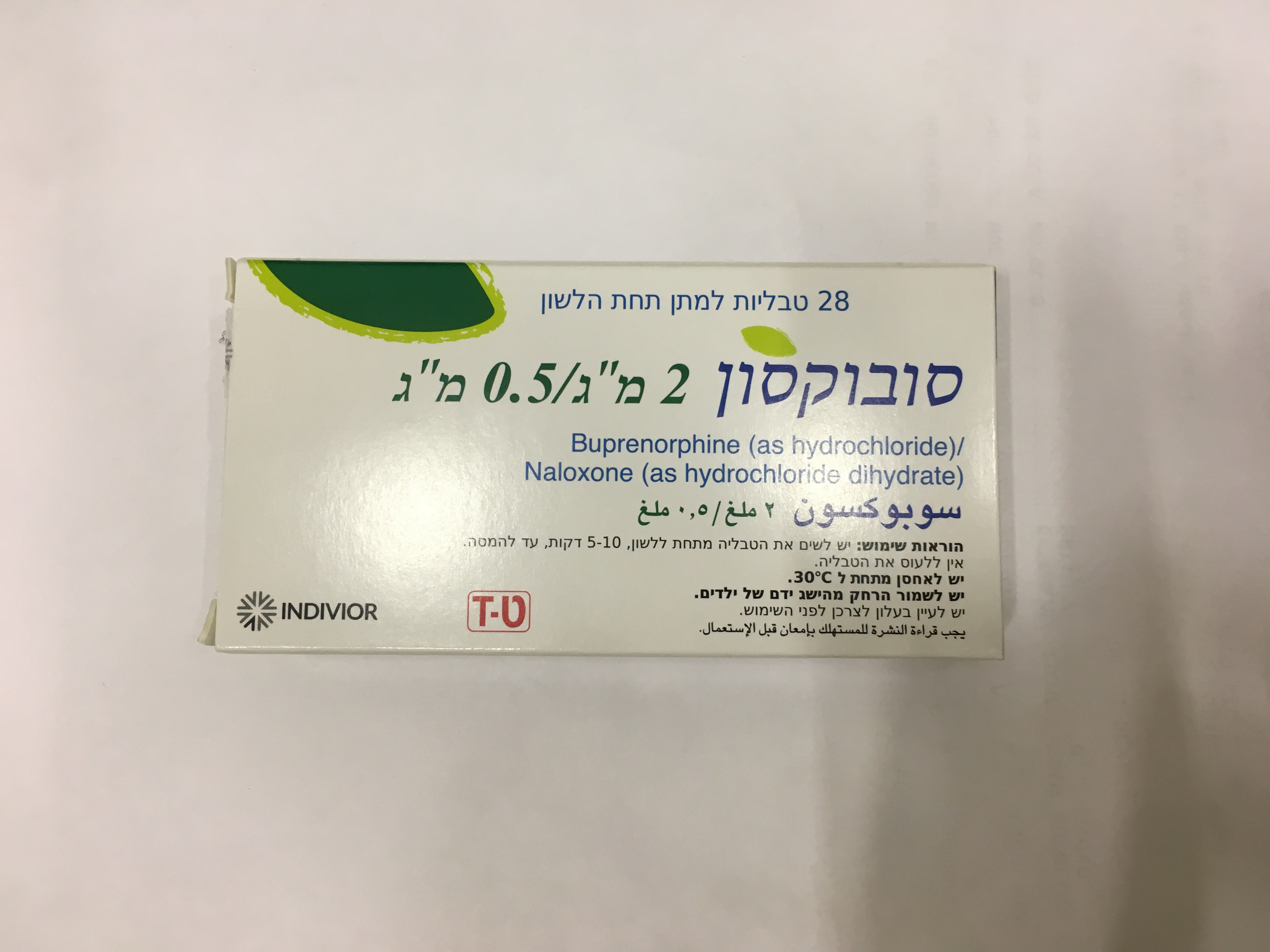
סובוקסון 2 מ"ג/ 0.5 מ"ג SUBOXONE 2 MG/0.5 MG (BUPRENORPHINE HYDROCHLORIDE, NALOXONE HYDROCHLORIDE DIHYDRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות למתן מתחת ללשון : TABLETS SUBLINGUAL
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Misuse, abuse and diversion Buprenorphine can be misused or abused in a manner similar to other opioids, legal or illicit. Some risks of misuse and abuse include overdose, spread of blood borne viral or localised and systemic infections, respiratory depression and hepatic injury. Buprenorphine misuse by someone other than the intended patient poses the additional risk of new drug dependent individuals using buprenorphine as the primary drug of abuse, and may occur if the medicinal product is distributed for illicit use directly by the intended patient or if it is not safeguarded against theft. Suboptimal treatment with buprenorphine/naloxone may prompt misuse by the patient, leading to overdose or treatment dropout. A patient who is underdosed with buprenorphine/naloxone may continue responding to uncontrolled withdrawal symptoms by self-medicating with opioids, alcohol or other sedative-hypnotics such as benzodiazepines. To minimize the risk of misuse, abuse and diversion, appropriate precautions should be taken when prescribing and dispensing buprenorphine, such as avoiding prescribing multiple refills early in treatment, and conducting patient follow-up visits with clinical monitoring that is appropriate for the patient's needs. Combining buprenorphine with naloxone in Suboxone is intended to deter misuse and abuse of the buprenorphine. Intravenous or intranasal misuse of Suboxone is expected to be less likely than with buprenorphine alone since the naloxone in this medicinal product can precipitate withdrawal in individual’s dependent on heroin, methadone, or other opioid agonists. Sleep-related breathing disorders Opioids can cause sleep-related breathing disorders including central sleep apnoea (CSA) and sleep- related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the total opioid dosage. Respiratory depression A number of cases of death due to respiratory depression have been reported, particularly when buprenorphine was used in combination with benzodiazepines (see section 4.5) or when buprenorphine was not used according to the prescribing information. Deaths have also been reported in association with concomitant administration of buprenorphine and other depressants such as alcohol or other opioids. If buprenorphine is administered to some non-opioid dependent individuals, who are not tolerant to the effects of opioids, potentially fatal respiratory depression may occur. This medicinal product should be used with care in patients with asthma or respiratory insufficiency (e.g. chronic obstructive pulmonary disease, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, pre-existing respiratory depression or kyphoscoliosis (curvature of spine leading to potential shortness of breath)). Buprenorphine/naloxone may cause severe, possibly fatal, respiratory depression in children and non- dependent persons in case of accidental or deliberate ingestion. Patients must be warned to store the blister safely, to never open the blister in advance, to keep them out of the reach of children and other household members, and not to take this medicinal product in front of children. An emergency unit should be contacted immediately in case of accidental ingestion or suspicion of ingestion. CNS depression Buprenorphine/naloxone may cause drowsiness, particularly when taken together with alcohol or central nervous system depressants (such as benzodiazepines, tranquilisers, sedatives or hypnotics) (see sections 4.5 and 4.7). Risk from concomitant use of sedative medicinal products such as benzodiazepines or related medicinal products Concomitant use of buprenorphine/naloxone and sedative medicinal products such as benzodiazepines or related medicinal products may result in sedation, respiratory depression, coma and death. Because of these risks, concomitant prescribing with these sedative medicinal products should be reserved for patients for whom alternative treatment options are not possible. If a decision is made to prescribe buprenorphine/naloxone concomitantly with sedative medicinal products, the lowest effective dose of the sedative medicines should be used, and the duration of treatment should be as short as possible. The patients should be followed closely for signs and symptoms of respiratory depression and sedation. In this respect, it is strongly recommended to inform patients and their caregivers to be aware of these symptoms (see section 4.5). Serotonin syndrome Concomitant administration of Suboxone and other serotonergic agents, such as MAO inhibitors, selective serotonin re-uptake inhibitors (SSRIs), serotonin norepinephrine re-uptake inhibitors (SNRIs) or tricyclic antidepressants may result in serotonin syndrome, a potentially life-threatening condition (see section 4.5). If concomitant treatment with other serotonergic agents is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases. Symptoms of serotonin syndrome may include mental-status changes, autonomic instability, neuromuscular abnormalities, and/or gastrointestinal symptoms. If serotonin syndrome is suspected, a dose reduction or discontinuation of therapy should be considered depending on the severity of the symptoms. Dependence Buprenorphine is a partial agonist at the µ (mu)-opiate receptor and chronic administration produces dependence of the opioid type. Studies in animals, as well as clinical experience, have demonstrated that buprenorphine may produce dependence, but at a lower level than a full agonist e.g. morphine. Abrupt discontinuation of treatment is not recommended as it may result in a withdrawal syndrome that may be delayed in onset. Hepatitis and hepatic events Cases of acute hepatic injury have been reported in opioid-dependent addicts both in clinical trials and in post marketing adverse reaction reports. The spectrum of abnormalities ranges from transient asymptomatic elevations in hepatic transaminases to case reports of hepatic failure, hepatic necrosis, hepatorenal syndrome, hepatic encephalopathy and death. In many cases the presence of pre-existing mitochondrial impairment (genetic disease, liver enzyme abnormalities, infection with hepatitis B or hepatitis C virus, alcohol abuse, anorexia, concomitant use of other potentially hepatotoxic medicinal product) and ongoing injecting drug use may have a causative or contributory role. These underlying factors must be taken into consideration before prescribing buprenorphine/naloxone and during treatment. When a hepatic event is suspected, further biological and etiological evaluation is required. Depending upon the findings, the medicinal product may be discontinued cautiously so as to prevent withdrawal symptoms and to prevent a return to illicit drug use. If the treatment is continued, hepatic function should be monitored closely. Precipitation of opioid withdrawal syndrome When initiating treatment with buprenorphine/naloxone, the physician must be aware of the partial agonist profile of buprenorphine and that it can precipitate withdrawal in opioid-dependent patients, particularly if administered less than 6 hours after the last use of heroin or other short-acting opioid, or if administered less than 24 hours after the last dose of methadone. Patients should be clearly monitored during the switching period from buprenorphine or methadone to buprenorphine/naloxone since withdrawal symptoms have been reported. To avoid precipitating withdrawal, induction with buprenorphine/naloxone should be undertaken when objective signs of withdrawal are evident (see section 4.2). Withdrawal symptoms may also be associated with sub-optimal dosing. Hepatic impairment The effects of hepatic impairment on the pharmacokinetics of buprenorphine and naloxone were evaluated in a post-marketing study. Both buprenorphine and naloxone are extensively metabolized in the liver, and plasma levels were found to be higher for both buprenorphine and naloxone in patients with moderate and severe hepatic impairment compared with healthy subjects. Patients should be monitored for signs and symptoms of precipitated opioid withdrawal, toxicity or overdose caused by increased levels of naloxone and/or buprenorphine. Baseline liver function tests and documentation of viral hepatitis status is recommended prior to commencing therapy. Patients who are positive for viral hepatitis, on concomitant medicinal products (see section 4.5) and/or have existing liver dysfunction are at greater risk of liver injury. Regular monitoring of liver function is recommended (see section 4.4). Buprenorphine/naloxone should be used with caution in patients with moderate hepatic impairment (see sections 4.3 and 5.2). In patients with severe hepatic insufficiency the use of buprenorphine/naloxone is contraindicated. Renal impairment Renal elimination may be prolonged since 30 % of the administered dose is eliminated by the renal route. Metabolites of buprenorphine accumulate in patients with renal failure. Caution is recommended when dosing patients with severe renal impairment (creatinine clearance <30 ml/min) (see sections 4.2 and 5.2). CYP 3A4 inhibitors Medicinal products that inhibit the enzyme CYP3A4 may give rise to increased concentrations of buprenorphine. A reduction of the buprenorphine/naloxone dose may be needed. Patients already treated with CYP3A4 inhibitors should have their dose of buprenorphine/naloxone titrated carefully since a reduced dose may be sufficient in these patients (see section 4.5). Class effects • Opioids may produce orthostatic hypotension in ambulatory patients. • Opioids may elevate cerebrospinal fluid pressure, which may cause seizures, so opioids should be used with caution in patients with head injury, intracranial lesions, in other circumstances where cerebrospinal pressure may be increased, or in patients with a history of seizure. • Opioids should be used with caution in patients with hypotension, prostatic hypertrophy or urethral stenosis. • Opioid-induced miosis, changes in the level of consciousness, or changes in the perception of pain as a symptom of disease may interfere with patient evaluation or obscure the diagnosis or clinical course of concomitant disease. • Opioids should be used with caution in patients with myxoedema, hypothyroidism, or adrenal cortical insufficiency (e.g., Addison's disease). • Opioids have been shown to increase intracholedochal pressure, and should be used with caution in patients with dysfunction of the biliary tract. • Opioids should be administered with caution to elderly or debilitated patients. The concomitant use of monoamine oxidase inhibitors (MAOI) might produce an exaggeration of the effects of opioids, based on experience with morphine (see section 4.5). Excipients This medicinal product contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine. This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’. Paediatric population Use in adolescents (age 15 - <18) Due to the lack of data in adolescents (age 15 - <18), patients in this age group should be more closely monitored during treatment.
Effects on Driving
4.7 Effects on ability to drive and use machines Buprenorphine/naloxone has minor to moderate influence on the ability to drive and use machines when administered to opioid dependent patients. This medicinal product may cause drowsiness, dizziness, or impaired thinking, especially during treatment induction and dose adjustment. If taken together with alcohol or central nervous system depressants, the effect is likely to be more pronounced (see sections 4.4 and 4.5). Patients should be cautioned about driving or operating hazardous machinery in case buprenorphine/naloxone may adversely affect their ability to engage in such activities.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
09/01/2013
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
