Quest for the right Drug
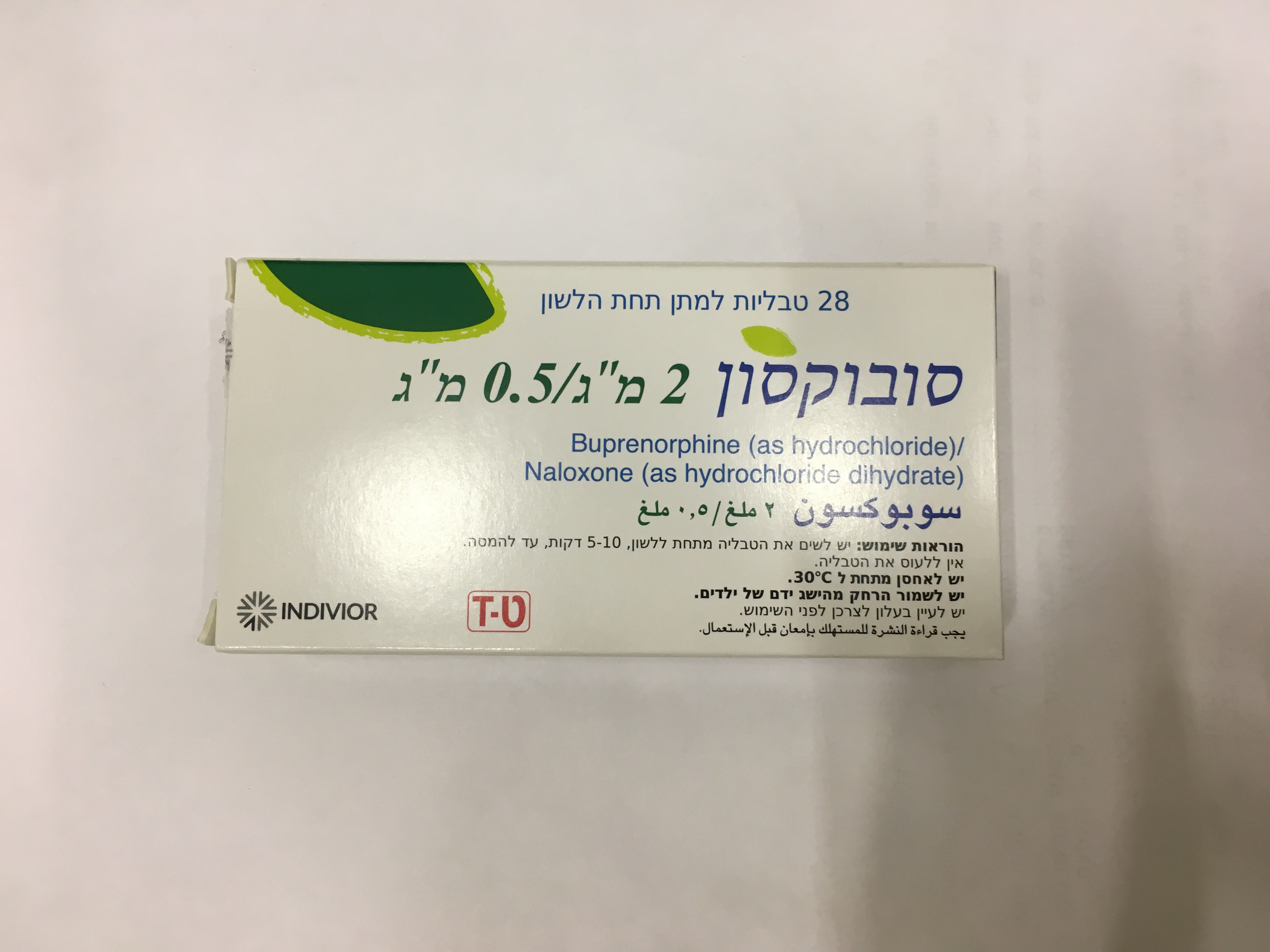
סובוקסון 2 מ"ג/ 0.5 מ"ג SUBOXONE 2 MG/0.5 MG (BUPRENORPHINE HYDROCHLORIDE, NALOXONE HYDROCHLORIDE DIHYDRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות למתן מתחת ללשון : TABLETS SUBLINGUAL
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pregnancy & Lactation : הריון/הנקה
4.6 Fertility, pregnancy and lactation Pregnancy There are no or limited amount of data from the use of buprenorphine/naloxone in pregnant women. Studies in animals have shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. Towards the end of pregnancy buprenorphine may induce respiratory depression in the newborn infant even after a short period of administration. Long-term administration of buprenorphine during the last three months of pregnancy may cause a withdrawal syndrome in the neonate. (e.g. hypertonia, neonatal tremor, neonatal agitation, myoclonus or convulsions). The syndrome is generally delayed for several hours to several days after birth. Due to the long half-life of buprenorphine, neonatal monitoring for several days should be considered at the end of pregnancy, to prevent the risk of respiratory depression or withdrawal syndrome in neonates. Furthermore, the use of buprenorphine/naloxone during pregnancy should be assessed by the physician. Buprenorphine/naloxone should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus. Breast-feeding It is unknown whether naloxone is excreted in human milk. Buprenorphine and its metabolites are excreted in human milk. In rat’s buprenorphine has been found to inhibit lactation. Therefore, breastfeeding should be discontinued during treatment with Suboxone. Fertility Animal studies have shown a reduction in female fertility at high doses (systemic exposure > 2.4 times the human exposure at the maximum recommended dose of 24 mg buprenorphine, based on AUC, see section 5.3).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
09/01/2013
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
