Quest for the right Drug
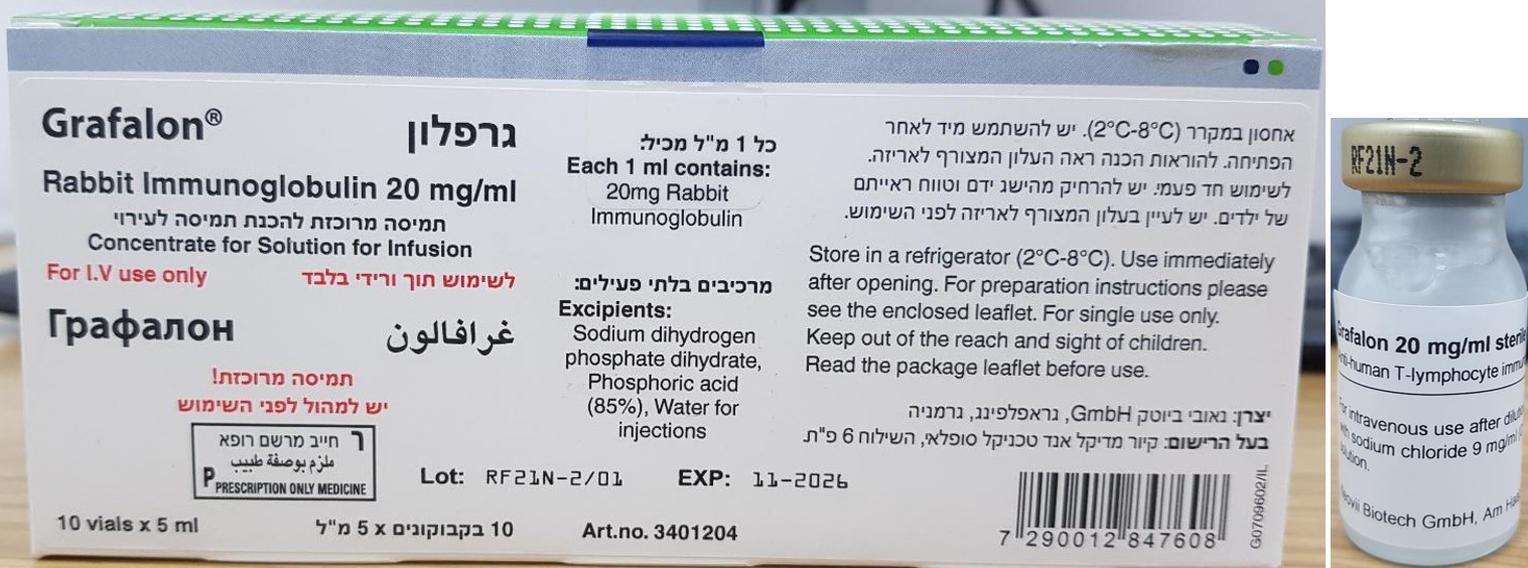
גרפלון GRAFALON (RABBIT IMMUNOGLOBULIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תרכיז להכנת תמיסה לאינפוזיה : CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: specific immunosuppressant, ATC code: L04AA04. Grafalon is a polyclonal anti-human-T-lymphocyte immunoglobulin derived from rabbits immunized with Jurkat cells, a human lymphoblastoid cell line. The expression of T-cell markers on Jurkat cells is consistent with the effects of Grafalon on lymphocytes. Grafalon has been found to contain antibodies against further surface antigens of Jurkat cells. Analysis of lymphocyte subsets in patients who received Grafalon, showed a decrease in lymphocyte subsets carrying surface proteins, which are expressed by the Jurkat cell line. Grafalon is cytotoxic against human lymphocytes. Available data show that activated lymphocytes are more susceptible. Grafalon did not activate T-cells (via CD3) or lymphocytes but inhibited activation of T-cells by an anti-CD3 antibody. Grafalon reduced migration of human melanoma cells by binding to adhesion molecules. Anti-adhesive properties (anti-LFA-1 and anti-ICAM-1 activity) might explain why addition of Grafalon diminished the vascular resistance of kidney vessels and reduced lymphocyte retention in the kidney when porcine kidneys were perfused with human lymphocytes incubated with or without Grafalon. Grafalon prolonged skin graft survival in rhesus monkeys. Immunosuppression was evident in this model and leukopenia and lymphopenia were observed. In cynomolgus monkeys, Grafalon had a beneficial effect on ischemia/reperfusion injury by inhibition of adhesion of lymphocytes and neutrophils. In renal transplant patients under standard therapy with Grafalon, leukocyte, and platelet counts decreased but returned to normal levels within 10 days after transplantation. Also counts of lymphocytes and lymphocyte subpopulations decreased significantly. A decrease in CD2, CD3, CD4 and CD8 count was observed. A return to levels within normal range was seen for CD8 but not for CD2, CD3 and CD4 in the first 20 post-operative days. The described effect of standard therapy with Grafalon on lymphocyte subpopulations and a persistent reversal of the CD4/CD8 ratio for up to 66 months were reported in patients after kidney transplantation. After a single high-dose of 9 mg/kg BW Grafalon, TNF- and IL-10 increased, while IL-12p40 slightly decreased and IL-12p70 was not stimulated. Stem cell transplantation study Results of a 2-year follow-up stem cell transplantation study with matched unrelated donor grafts showed that the incidence of acute graft-versus-host disease (aGVHD), chronic GVHD (cGVHD) and mortality due to GVHD was decreased in patients receiving Grafalon in addition to standard prophylaxis compared to patients receiving only GVHD standard prophylaxis. Methods: The study was a Phase 3 prospective, open-label randomised, multicentre study conducted in 10 countries at 31 centres across Europe, enrolling 202 adult patients with haematological malignancies. One group (98 patients) received standard prophylaxis with cyclosporin and methotrexate, whilst the other group (103 patients) received Grafalon in addition to standard prophylaxis; 20 mg/kg of Grafalon was administered on Day -3, Day -2 and Day -1 prior to SCT. 201 patients who underwent transplantation with peripheral blood (n = 164; 82%) or bone marrow (n = 37; 18%) grafts from matched unrelated donors after myeloablative conditioning were included in the study. The primary endpoint was early treatment failure: Severe aGVHD grade III - IV or death within 100 days of transplantation. Results: The addition of Grafalon to standard prophylaxis resulted in a decreased incidence of all forms of GVHD: aGVHD (severity groups I - IV, II - IV and III - IV) and cGVHD (limited and extensive form). There were no differences between treatment groups with regard to relapse, transplant-related mortality, and overall survival. Primary endpoint: The incidence of early treatment failure was 21.4%, compared to 34.7% in the control group (adjusted odds ratio 0.56, CI [0.28 - 1.11]; p = 0.0983). The cumulative incidence of aGVHD grade III - IV was 11.7% in the Grafalon group versus 25.5% in the control group (adjusted hazard ratio [HR] 0.48, CI [0.24 - 0.96]; p = 0.0392). The cumulative incidence of aGVHD grade II - IV was 33.0% in the Grafalon group versus 52.0% in the control group (adjusted HR 0.55, CI [0.35 - 0.85]; p = 0.0077). The 2-year post-transplantation follow-up incidence of extensive chronic GVHD with Grafalon was 12.2% versus 45.0% in the control group without Grafalon prophylaxis (adjusted HR 0.196, CI [0.10 - 0.39]; p < 0.0001). Figure 1 Relative Risk of Grafalon prophylaxis vs. control group without Grafalon prophylaxis for primary and secondary efficacy parameters (point estimator and 95% CI)
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Grafalon is administered intravenously and is therefore 100% bioavailable. Grafalon is subject to protein metabolism as are other bodily proteins. The half-life of Grafalon is approximately 14 days (in case of a dosage of 4 mg/kg BW/d over 7 days) and varies from 4 to 45 days depending on the dose and duration of administration. Literature studies have shown that T-cell specific antibodies were eliminated faster than total rabbit IgG. Pharmacokinetic data have been obtained from the toxicokinetic sections of the toxicology studies. Grafalon is absorbed rapidly and is eliminated slowly. Systemic exposure was proportionate at all dose levels, increased with repeated dosing, without gender differences. No drug-drug interactions with prednisolone were seen.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
