Quest for the right Drug
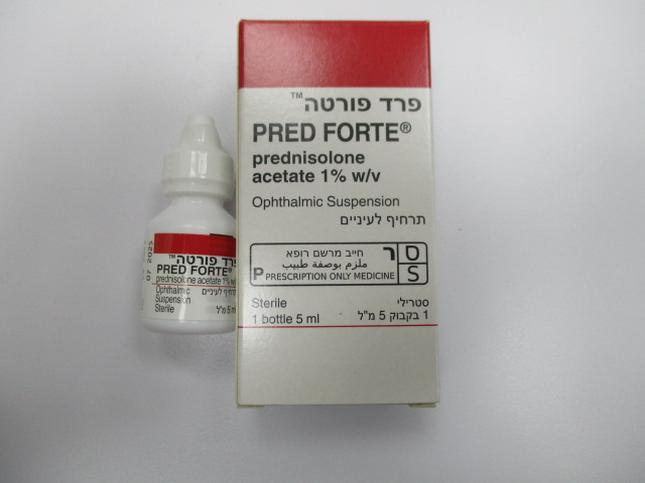
פרד פורטה PRED-FORTE (PREDNISOLONE ACETATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
עיני : OCULAR
צורת מינון:
תרחיף לעין : EYE DROPS, SUSPENSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Acute purulent infections of the eye may be masked or enhanced by the use of topical steroids. Pred Forte contains no antimicrobial agent. If infection is present, appropriate measures must be taken to counteract the infective organisms. Prolonged use may also suppress the host immune response and thus increase the hazard of secondary ocular infections. Fungal infections of the cornea have been reported coincidentally with long-term steroid application and fungal invasion may be suspected in any persistent corneal ulceration where a steroid has been used, or is in use. Various ocular diseases and long-term use of topical corticosteroids have been known to cause corneal or scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation. The preservative in Pred Forte, benzalkonium chloride, may be absorbed by and cause discoloration of soft contact lenses. Patients wearing soft contact lenses should be instructed to remove contact lenses prior to administration of the solution and wait at least 15 minutes after instilling Pred Forte before reinserting soft contact lenses. Use of intraocular steroids may prolong the course and may exacerbate the severity of many viral infections on the eye (including herpes simplex). Patients with a history of herpes simplex keratitis should be treated with caution. Use of steroid medication in the presence of stromal herpes simplex requires caution and should be followed by frequent, mandatory, slit-lamp microscopy. Prolonged use of topical corticosteroids may cause an increase in intraocular pressure in certain individuals. This may result in glaucoma with damage to the optic nerve with resultant defects in visual acuity and visual fields. Steroids should be used with caution in the presence of glaucoma. It is advisable that intraocular pressure be checked frequently during treatment with Pred Forte. Eye drops containing corticosteroids should not be used for more than 7 days except under strict ophthalmic supervision with regular checks for intraocular pressure. Posterior subcapsular cataract formation has been reported after heavy or protracted use of topical ophthalmic corticosteroids. The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation. Systemic adverse events may occur with extensive use of topical steroids; punctal occlusion may be recommended (see Section 4.2). The possibility of adrenal suppression should be considered with prolonged, frequent, use of high dose topical steroids, particularly in infants and children. To prevent eye injury or contamination, care should be taken to avoid touching the bottle tip to the eye or to any other surface. Visual disturbance Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids.
Effects on Driving
4.7 Effects on ability to drive and use machines Pred Forte may cause short-lasting blurring of vision upon instillation. If affected, the patient should not use machinery/electric tools or drive until vision has returned to normal.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/2009
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
04.08.20 - עלון לצרכן אנגלית 04.08.20 - עלון לצרכן עברית 04.08.20 - עלון לצרכן ערבית 11.05.23 - עלון לצרכן עברית 27.09.23 - עלון לצרכן אנגלית 27.09.23 - עלון לצרכן עברית 27.09.23 - עלון לצרכן ערבית 12.01.24 - עלון לצרכן עברית 03.04.24 - עלון לצרכן עברית 13.04.24 - עלון לצרכן אנגלית 13.04.24 - עלון לצרכן ערבית 04.08.20 - החמרה לעלון 12.01.24 - החמרה לעלון 03.04.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
פרד פורטה