Quest for the right Drug
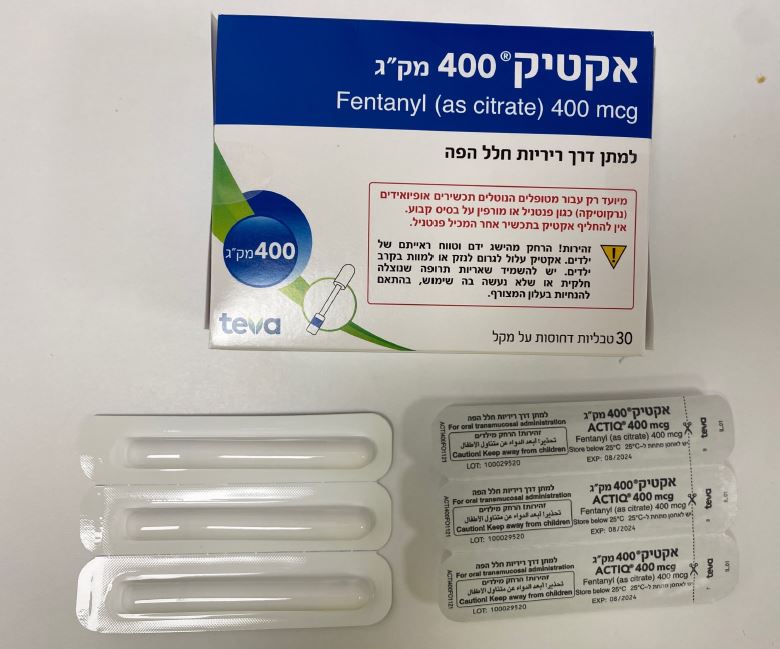
אקטיק 400 מק"ג ACTIQ 400 MCG (FENTANYL AS CITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי בחלל הפה : ORAL TRANSMUCOSAL
צורת מינון:
טבליות דחוסות על מקל : COMPRESSED TABLET ON A HANDLE
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Posology In order to minimise the risks of opioid-related adverse reactions and to identify the “successful” dose, it is imperative that patients be monitored closely by health professionals during the titration process. ACTIQ is not interchangeable on a mcg to mcg basis with other short-acting fentanyl products that are indicated for the use of breakthrough cancer pain, as the pharmacokinetic profiles and/or dosing schedules of these products are significantly different. Patients should be instructed not to use more than one short-acting fentanyl product concurrently for the treatment of breakthrough cancer pain, and to dispose of any fentanyl product prescribed for breakthrough pain (BTP) when switching to ACTIQ. The number of ACTIQ strengths available to the patient at any time should be minimised to prevent confusion and potential overdose. Any unused ACTIQ units that the patient no longer requires must be disposed of properly. Patients must be reminded of the requirements to keep ACTIQ stored in a location away from children. Adults Dose titration and maintenance therapy ACTIQ should be individually titrated to a “successful” dose that provides adequate analgesia and minimises adverse reactions. In clinical trials the successful dose of ACTIQ for breakthrough pain was not predicted from the daily maintenance dose of opioid. a) Titration Before patients are titrated with ACTIQ, it is expected that their background persistent pain will be controlled by use of opioid therapy and that they are typically experiencing no more than 4 episodes of breakthrough pain per day. The initial dose of ACTIQ used should be 200 micrograms, titrating upwards as necessary through the range of available dosage strengths (200, 400, 600, 800, 1,200 and 1,600 micrograms). Patients should be carefully monitored until a dose is reached that provides adequate analgesia with acceptable adverse reactions using a single dosage unit per episode of breakthrough pain. This is defined as the successful dose. During titration, if adequate analgesia is not obtained within 30 minutes after starting the first unit (i.e. 15 minutes after the patient completes consumption of a single ACTIQ unit), a second ACTIQ unit of the same strength may be consumed. No more than two ACTIQ units should be used to treat any individual pain episode. At 1600 micrograms, a second dose is only likely to be required by a minority of patients. If treatment of consecutive breakthrough pain episodes requires more than one dosage unit per episode, an increase in dose to the next higher available strength should be considered. ACTIQ Titration Process Start dosing at 200 micrograms 1. Patient consumes ACTIQ over 15 minutes 2. Patient waits 15 minutes. If inadequate analgesia, patient consumes a second ACTIQ unit of the same strength 3. Patient tries this ACTIQ dose for consecutive episodes of breakthrough pain Did the patient achieve adequate pain relief with one ACTIQ unit? Yes No Successful dose Increase dose to next determined strength* *Available dosage strengths include: 200, 400, 600, 800, 1200, and 1600 micrograms b) Maintenance Once a successful dose has been established (i.e., on average, an episode is effectively treated with a single unit), patients should be maintained on this dose and should limit consumption to a maximum of four ACTIQ units per day. Patients should be monitored by a health professional to ensure that the maximum consumption of four units of ACTIQ per day is not exceeded. Dose re-adjustment The maintenance dose of ACTIQ should be increased when an episode is not effectively treated with a single unit for several consecutive BTP episodes. For dose-readjustment the same principles apply as outlined for dose titration (see above). If more than four episodes of breakthrough pain are experienced per day the dose of the maintenance opioid therapy used for persistent pain should be re-evaluated. If the dose of the maintenance opioid therapy is increased, the dose of ACTIQ to treat breakthrough pain may need to be reviewed. In absence of adequate pain control, the possibility of hyperalgesia, tolerance and progression of underlying disease should be considered (see section 4.4). It is imperative that any dose re-titration of any analgesic is monitored by a health professional. Treatment duration and goals Before initiating treatment with ACTIQ, a treatment strategy including treatment duration and treatment goals, and a plan for end of the treatment, should be agreed together with the patient, in accordance with pain management guidelines. During treatment, there should be frequent contact between the physician and the patient to evaluate the need for continued treatment, consider discontinuation and to adjust dosages if needed. In absence of adequate pain control, the possibility of hyperalgesia, tolerance and progression of underlying disease should be considered (see section 4.4). ACTIQ should not be used longer than necessary. Discontinuation of therapy ACTIQ should be discontinued immediately if the patient no longer experiences breakthrough pain episodes. The treatment for the persistent background pain should be kept as prescribed. If discontinuation of all opioid therapy is required, the patient must be closely followed by the doctor as gradual downward opioid titration is necessary in order to avoid the possibility of abrupt withdrawal effects. Use in the elderly Elderly patients have been shown to be more sensitive to the effects of fentanyl when administered intravenously. Therefore dose titration needs to be approached with particular care. In the elderly, elimination of fentanyl is slower and the terminal elimination half-life is longer, which may result in accumulation of the active substance and to a greater risk of undesirable effects. Formal clinical trials with ACTIQ have not been conducted in the elderly. It has been observed, however, in clinical trials that patients over 65 years of age required lower doses of ACTIQ for successful relief of breakthrough pain. Use in patients with hepatic or renal impairment Special care should be taken during the titration process in patients with kidney or liver dysfunction (see section 4.4). Paediatric population Adolescents aged 16 years and above: Follow adult dosage. Actiq is not indicated for children and adolescents under 16 years. Method of administration ACTIQ is intended for oromucosal administration, and therefore should be placed in the mouth against the cheek and should be moved around the mouth using the applicator, with the aim of maximising the amount of mucosal exposure to the product. The ACTIQ unit should be sucked, not chewed, as absorption of fentanyl via the buccal mucosa is rapid in comparison with systemic absorption via the gastrointestinal tract. Water may be used to moisten the buccal mucosa in patients with a dry mouth. The ACTIQ unit should be consumed over a 15 minute period. If signs of excessive opioid effects appear before the ACTIQ unit is fully consumed it should be immediately removed, and consideration given to decreasing future dosages.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול בכאב מתפרץ בחולי סרטן. התחלת הטיפול בתרופה יעשה על פי מרשם של רופא מומחה באונקולוגיה, או בהמטואונקולוגיה או בכאב או בנוירולוגיה או בהרדמה
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| לטיפול בכאב מתפרץ בחולי סרטן | 01/03/2008 |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2008
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
14.07.21 - עלון לצרכן אנגלית 23.02.22 - עלון לצרכן אנגלית 14.07.21 - עלון לצרכן עברית 15.08.22 - עלון לצרכן עברית 14.07.21 - עלון לצרכן ערבית 23.02.22 - עלון לצרכן ערבית 12.10.22 - עלון לצרכן ערבית 14.11.22 - עלון לצרכן אנגלית 14.11.22 - עלון לצרכן עברית 14.11.22 - עלון לצרכן ערבית 15.08.23 - עלון לצרכן אנגלית 15.08.23 - עלון לצרכן 15.08.23 - עלון לצרכן אנגלית 21.08.23 - עלון לצרכן אנגלית 21.08.23 - עלון לצרכן עברית 21.08.23 - עלון לצרכן ערבית 21.06.24 - עלון לצרכן אנגלית 21.06.24 - עלון לצרכן עברית 21.06.24 - עלון לצרכן ערבית 17.01.19 - החמרה לעלון 22.10.19 - החמרה לעלון 06.07.20 - החמרה לעלון 10.01.21 - החמרה לעלון 14.07.21 - החמרה לעלון 20.10.21 - החמרה לעלון 03.01.22 - החמרה לעלון 15.08.22 - החמרה לעלון 12.10.22 - החמרה לעלון 15.06.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אקטיק 400 מק"ג