Quest for the right Drug
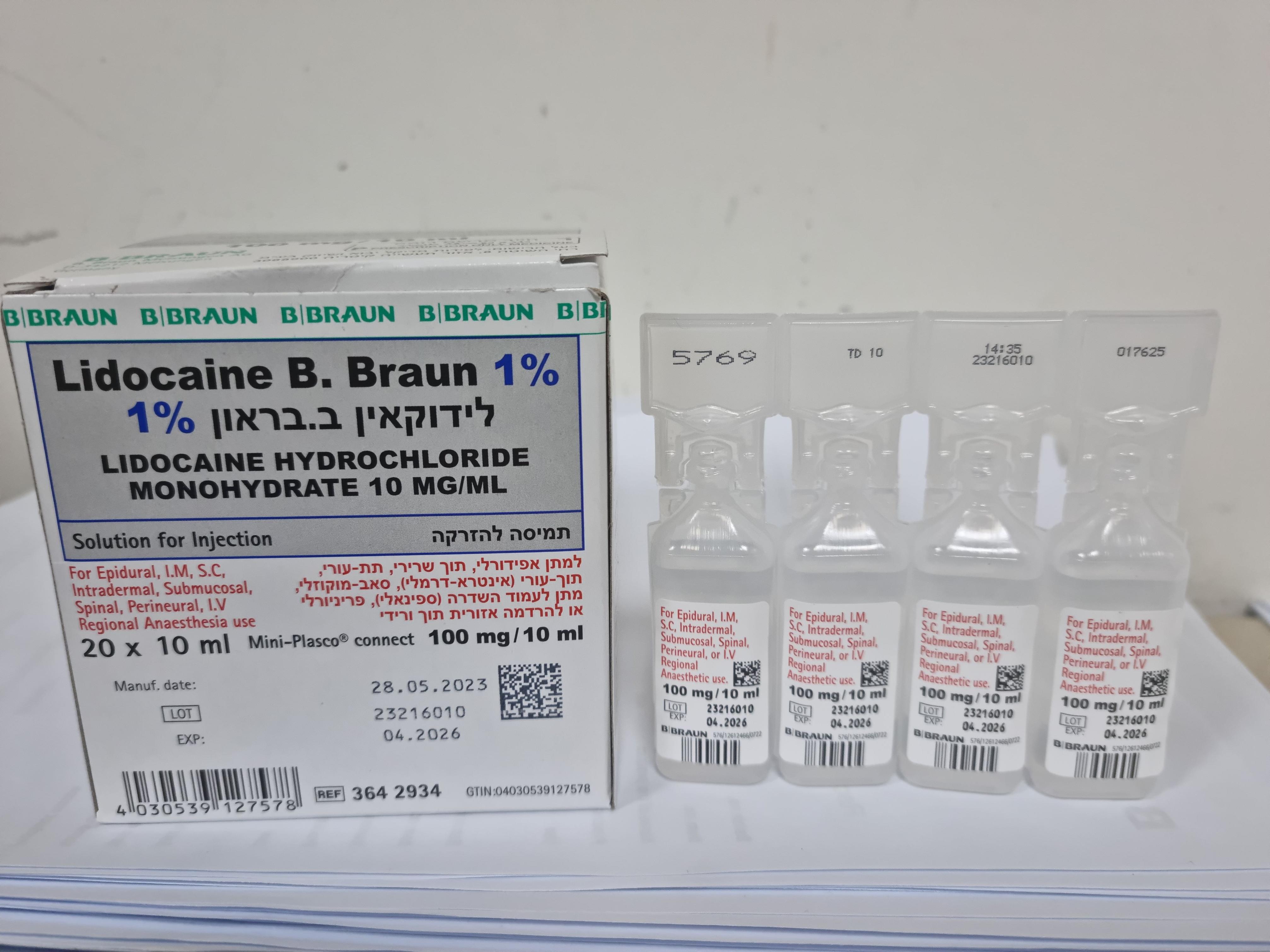
לידוקאין ב.בראון % 1 LIDOCAINE B.BRAUN 1 % (LIDOCAINE HYDROCHLORIDE MONOHYDRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, אפידורל, תת-עורי, תוך-עורי, הזרקה תת-רירית, שדרתי, לסביבת העצב, : I.M, EPIDURAL, S.C, INTRADERMAL, SUBMUCOSAL, SPINAL, PERINEURAL, I.V REGIONAL ANAESTHESIA
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects General The frequency and severity of the undesirable effects of lidocaine depend upon the dose, the method of administration and the patient‘s individual sensitivity. Symptoms of local toxicity may occur after the administration of lidocaine. Systemic adverse effects may be expected at plasma concentrations of lidocaine exceeding 5-10 mg/l. They become manifest in the form of both CNS symptoms and cardiovascular symptoms (see also section 4.9). The possible undesirable effects after administration of lidocaine as local anaesthetic are largely the same as those produced by other amide-type local anaesthetics. Listing of undesirable effects Definition of frequency terms used in this section: Very common: (≥ 1/10) Common: (≥ 1/100 to < 1/10) Uncommon: (≥ 1/1,000to < 1/100) Rare: (≥ 1/10,000 to < 1/1,000) Very rare (< 1/10,000) Not known: (frequency cannot be estimated from the available data) Local and regional anaesthesia Blood and the lymphatic system disorders Not known: Methaemoglobinaemia Immune system disorders Rare: Anaphylactic reactions manifesting as urticaria, oedema, bronchospasm, respiratory distress and circulatory symptoms up to anaphylactic shock. Nervous system disorders Common: Transient neurological symptoms especially after spinal and epidural anaesthesia, (up to 5 days). Rare: Neurological complications following central nervous blocks – mainly spinal anaesthesia – such as persistent anaesthesia,paraesthesia, paresis up to paraplegia, Cauda equina syndrome (i.e. bilateral leg weakness up to paraplegia, saddle anaesthesia,urinary retention and fecal incontinence), headache accompanied by tinnitus and photophobia Cranial nerve lesions, neurosensory deafness (if administered in head and neck regions). Horner’s syndrome, associated with epidural anaesthesia or regional applications in the head/neck region. Gastrointestinal disorder Very common: Nausea, vomiting Injury, poisoning and procedural complications Rare: Trauma, transient radicular irritation due to spinal anaesthesia, compression of the spinal cord after development of haematoma General disorders and administration site conditions Rare: Shivering (after epidural use) Information on particular undesirable effects none Paediatric population Frequency, type and severity of adverse reactions in children are expected to be the same as in adults. Elderly patients In elderly patients the incidence of undesirable effects may be increased (see section 4.4). Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. . Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form (https://sideeffects.health.gov.il))

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
