Quest for the right Drug
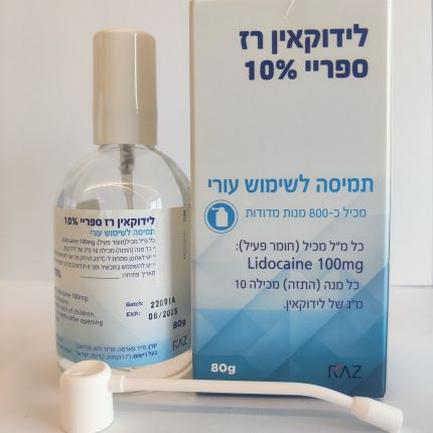
לידוקאין רז ספריי 10 % LIDOCAINE RAZ SPRAY 10 % (LIDOCAINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
עורי : CUTANEOUS
צורת מינון:
תמיסה : SOLUTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Any unused medicinal product or waste material should be Pharmacotherapeutic group (ATC Code): N01B B02 disposed of in accordance with local requirements. Lidocaine, like other local anaesthetics, causes a 7. Registration holder reversible blockade of impulse propagation along nerve fibres by preventing the inward movement of sodium ions RAZ PHARMACEUTICS LTD., through the nerve membrane. Local anaesthetics of the 31 GESHER HAETZ ST., INDUSTRIAL PARK amide-type are thought to act within the sodium channels EMEK HEFER, ISRAEL of the nerve membrane. 8. Manufacturer Local anaesthetic drugs may also have similar effects on excitable membranes in the brain and myocardium. If Sidefarma, Portugal. excessive amounts of drug reach the systemic circulation rapidly, symptoms and signs of toxicity will appear, 9. MARKETING AUTHORISATION NUMBER emanating from the central nervous and cardiovascular 170-02-36184-00 systems. Central nervous system toxicity usually precedes the Revised in March 2023 according to MOH’s guidelines. cardiovascular effects since it occurs at lower plasma RAZS0028-01 concentrations. Direct effects of local anaesthetics on the heart include slow conduction, negative inotropism and eventually cardiac arrest.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Absorption Lidocaine is absorbed following topical administration to mucous membranes; its rate and extent of absorption being dependent upon the concentration and total dose administered, the specific site of application, and duration of exposure. In general, the rate of absorption of local anaesthetic agents following topical application is most rapid after intratracheal and bronchial administration. Lidocaine is also well absorbed from the gastrointestinal tract, although little of the intact drug appears in the circulation because of biotransformation in the liver. Distribution The plasma protein binding of lidocaine is dependent on the drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 microgram of free base per ml, 60 to 80 percent of lidocaine is protein- bound. Binding is also dependent on the plasma concentration of the alpha-1-acid glycoprotein. Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion. Biotransformation Lidocaine is metabolised rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/ toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6-dimethylaniline. Elimination The elimination half-life of lidocaine following an intravenous bolus injection is typically 1.5 to 2.0 hours. Because of the rapid rate at which lidocaine is metabolised, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites. Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6.0 microgram free base per ml.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
