Quest for the right Drug
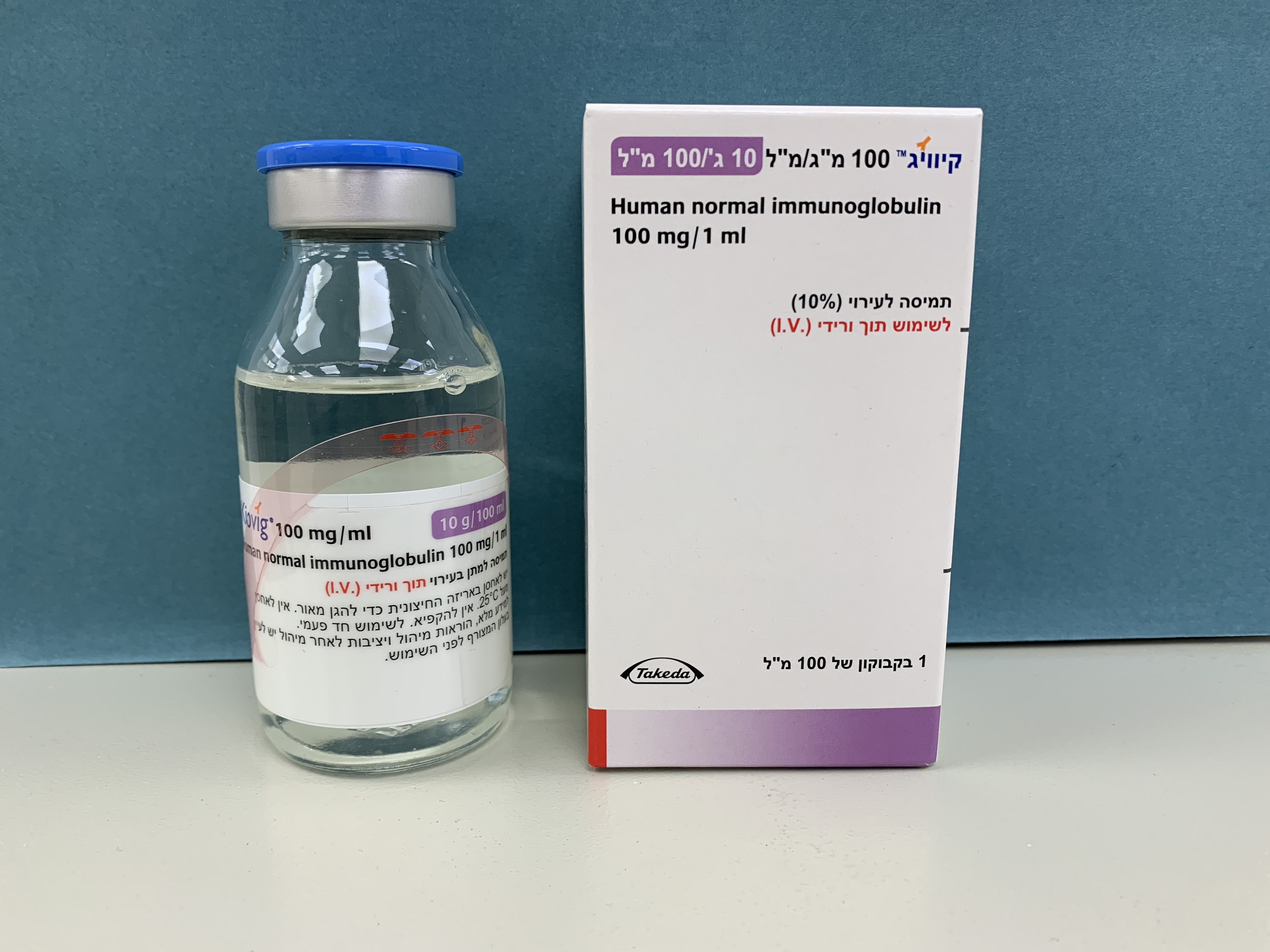
קיוויג 100 מ"ג/מ"ל KIOVIG 100 MG/ML (IMMUNOGLOBULINS, NORMAL HUMAN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה לאינפוזיה : SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Adverse reactions such as chills, headache, dizziness, fever, vomiting, allergic reactions, nausea, arthralgia, low blood pressure and moderate low back pain may occur occasionally. Rarely human normal immunoglobulins may cause a sudden fall in blood pressure and, in isolated cases, anaphylactic shock, even when the patient has shown no hypersensitivity to previous administration. Cases of reversible aseptic meningitis and rare cases of transient cutaneous reactions (including cutaneous lupus erythematosus - frequency unknown) have been observed with human normal immunoglobulin. Reversible haemolytic reactions have been observed in patients, especially those with blood groups A, B, and AB. Rarely, haemolytic anaemia requiring transfusion may develop after high-dose IVIg treatment (see also Section 4.4). Increase in serum creatinine level and/or acute renal failure have been observed. Very rarely: Thromboembolic reactions such as myocardial infarction, stroke, pulmonary embolism, and deep vein thromboses. Cases of Transfusion Related Acute Lung Injury (TRALI). Tabulated list of adverse reactions The tables presented below are according to the MedDRA system organ classification (SOC and Preferred Term Level). Table 1 shows the adverse reactions from clinical trials and Table 2 shows the post-marketing ARs. Frequencies have been evaluated according to the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. Table 1 Frequency of Adverse Reactions (ADRs) in clinical studies with KIOVIG MedDRA System Organ Class Adverse reaction Frequency (SOC) Bronchitis, nasopharyngitis Common Table 1 Frequency of Adverse Reactions (ADRs) in clinical studies with KIOVIG MedDRA System Organ Class Adverse reaction Frequency (SOC) Infections and Chronic sinusitis, fungal infection, infection, Uncommon infestations kidney infection, sinusitis, upper respiratory tract infection, urinary tract infection, bacterial urinary tract infection, meningitis aseptic Blood and lymphatic Anaemia, lymphadenopathy Common system disorders Immune system Hypersensitivity, anaphylactic reaction Uncommon disorders Endocrine disorders Thyroid disorder Uncommon Metabolism and Decreased appetite Common nutrition disorders Psychiatric disorders Insomnia, anxiety Common Irritability Uncommon Nervous system Headache Very common disorders Dizziness, migraine, paresthesia, hypoesthesia Common Amnesia, dysarthria, dysgeusia, balance Uncommon disorder, tremor Eye disorders Conjunctivitis Common Eye pain, eye swelling Uncommon Ear and labyrinth Vertigo, fluid in middle ear Uncommon disorders Cardiac disorders Tachycardia Common Vascular disorders Hypertension Very common Flushing Common Peripheral coldness, phlebitis Uncommon Respiratory, thoracic Cough, rhinorrhoea, asthma, nasal congestion, Common and mediastinal oropharyngeal pain, dyspnea disorders Oropharyngeal swelling Uncommon Gastrointestinal Nausea Very common disorders Diarrhoea, vomiting, abdominal pain, dyspepsia Common Abdominal distension Uncommon Skin and subcutaneous Rash Very common tissue disorders Contusion, pruritus, urticaria, dermatitis, Common erythema Angioedema, acute urticaria, cold sweat, Uncommon photosensitivity reaction, night sweats, hyperhidrosis Musculoskeletal and Back pain, arthralgia, pain in extremity, Common connective tissue myalgia, muscle spasms, muscular weakness disorders Muscle twitching Uncommon Renal and urinary Proteinuria Uncommon disorders General disorders and Local reactions (e.g. infusion site Very common administration site pain/swelling/reaction/pruritus), pyrexia, conditions fatigue Table 1 Frequency of Adverse Reactions (ADRs) in clinical studies with KIOVIG MedDRA System Organ Class Adverse reaction Frequency (SOC) Chills, edema, influenza-like illness, chest Common discomfort, chest pain, asthenia, malaise, rigors Chest tightness, feeling hot, burning sensation, Uncommon swelling Investigations Blood cholesterol increased, blood creatinine Uncommon increased, blood urea increased, white blood cell count decreased, alanine aminotransferase increased, haematocrit decreased, red blood cell count decreased, respiratory rate increased Table 2 Post-Marketing Adverse Reactions (ARs) MedDRA System Organ Class Adverse reaction Frequency (SOC) Blood and lymphatic Haemolysis Not known system disorders Immune system Anaphylactic shock Not known disorders Nervous system Transient ischemic attack, cerebral vascular Not known disorders accident Cardiac disorders Myocardial infarction Not known Vascular disorders Hypotension, deep vein thrombosis Not known Respiratory, thoracic Pulmonary embolism, pulmonary edema Not known and mediastinal disorders Investigations Coombs direct test positive, oxygen Not known saturation decreased Injury, poisoning and Transfusion-related acute lung injury Not known procedural complications Description of selected adverse reactions Muscle twitching and weakness were reported only in patients with MMN. Paediatric population Frequency, type and severity of adverse reactions in children are the same as in adults. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il For safety with respect to transmissible agents, see section 4.4.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול במקרים האלה: א. חסר חיסוני ראשוני (חולים עם פגיעה ראשונית בייצור נוגדנים כגון אגמגלובולינמיה או היפוגמגלובוילינמיה, ITP (Idiopathic thrombocytopenic purpura)); ב. חסר חיסוני ספציפי, מניעה או טיפול בחצבת, הפטיטיס A ויראלית; ג. CIDP – Chronic inflammatory demyelineating polyneuropathy; ד.טיפול בחולי לוקמיה מסוג CLL הסובלים מהיפוגלמגלובולינמיה משנית חמורה וזיהומים חוזרים.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| טיפול בחולי לוקמיה מסוג CLL הסובלים מהיפוגלמגלובולינמיה משנית חמורה וזיהומים חוזרים. | 01/01/1995 | |||
| CIDP – Chronic inflammatory demyelineating polyneuropathy; | 01/01/1995 | |||
| חסר חיסוני ספציפי, מניעה או טיפול בחצבת, הפטיטיס A ויראלית | 01/01/1995 | |||
| חסר חיסוני ראשוני (חולים עם פגיעה ראשונית בייצור נוגדנים כגון אגמגלובולינמיה או היפוגמגלובולינמיה, ITP (Idiopathic thrombocytopenic purpura)); | 01/01/1995 |
שימוש לפי פנקס קופ''ח כללית 1994
Primary immunodeficiency (patients with primary defective antibody synthesis such as agammaglobulinemia or hypogammaglobulinemia, idiopathic thrombocytopenic purpura (ITP)
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
