Quest for the right Drug
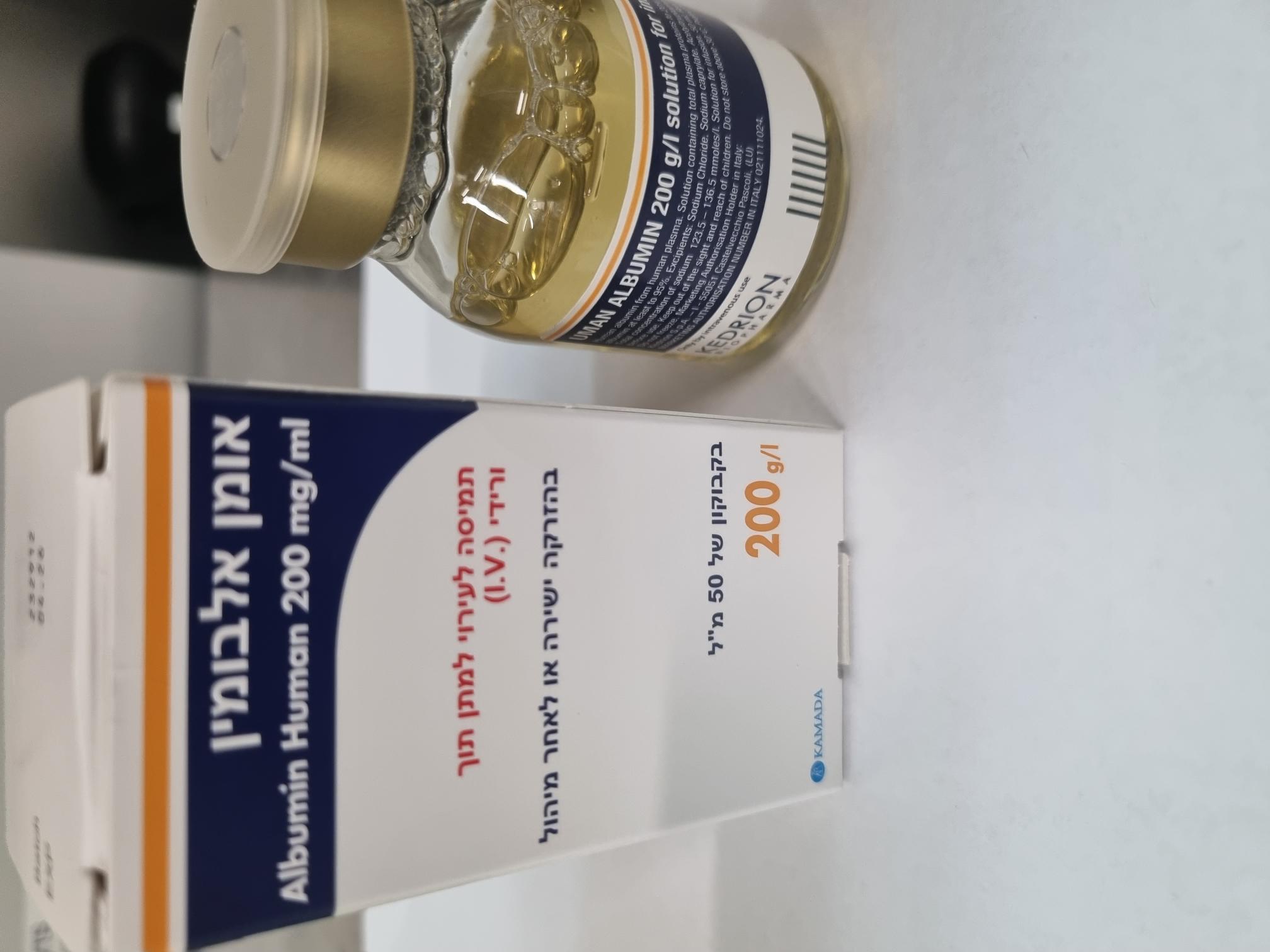
אומן אלבומין UMAN ALBUMIN (ALBUMIN HUMAN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה לאינפוזיה : SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: plasma substitutes and plasma protein fractions, ATC code: B05AA01. Human albumin accounts quantitatively for more than half of the total protein in the plasma and represents about 10% of the protein synthesis activity of the liver. Physicochemical data: Human albumin 200 g/l has a corresponding hyperoncotic effect. The most important physiological function of albumin results from its contribution to oncotic pressure of the blood and transport functions. Albumin stabilises circulating blood volume and is a carrier of hormones, enzymes, medicinal products and toxins. Paediatric population No specific studies of efficacy and safety are available on paediatric population.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Under normal conditions, the total exchangeable albumin pool is 4-5 g/kg body weight, of which 40-45% is present intravascularly and 55-60% in the extravascular space. Increased capillary permeability will alter albumin kinetics and abnormal distribution may occur in conditions such as severe burns or septic shock. Under normal conditions, the average half-life of albumin is about 19 days. The balance between synthesis and breakdown is normally achieved by feed-back regulation. Elimination is predominantly intracellular and due to lysosome proteases. In healthy subjects, less than 10% of infused albumin leaves the intravascular compartment during the first 2 hours following infusion. There is a considerable individual variation in the effect on plasma volume. In some patients the plasma volume can remain increased for some hours. However, in critically ill patients, albumin can leak out of the vascular space in substantial amounts at an unpredictable rate. Paediatric population No specific studies of efficacy and safety are available on paediatric population

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
