Quest for the right Drug
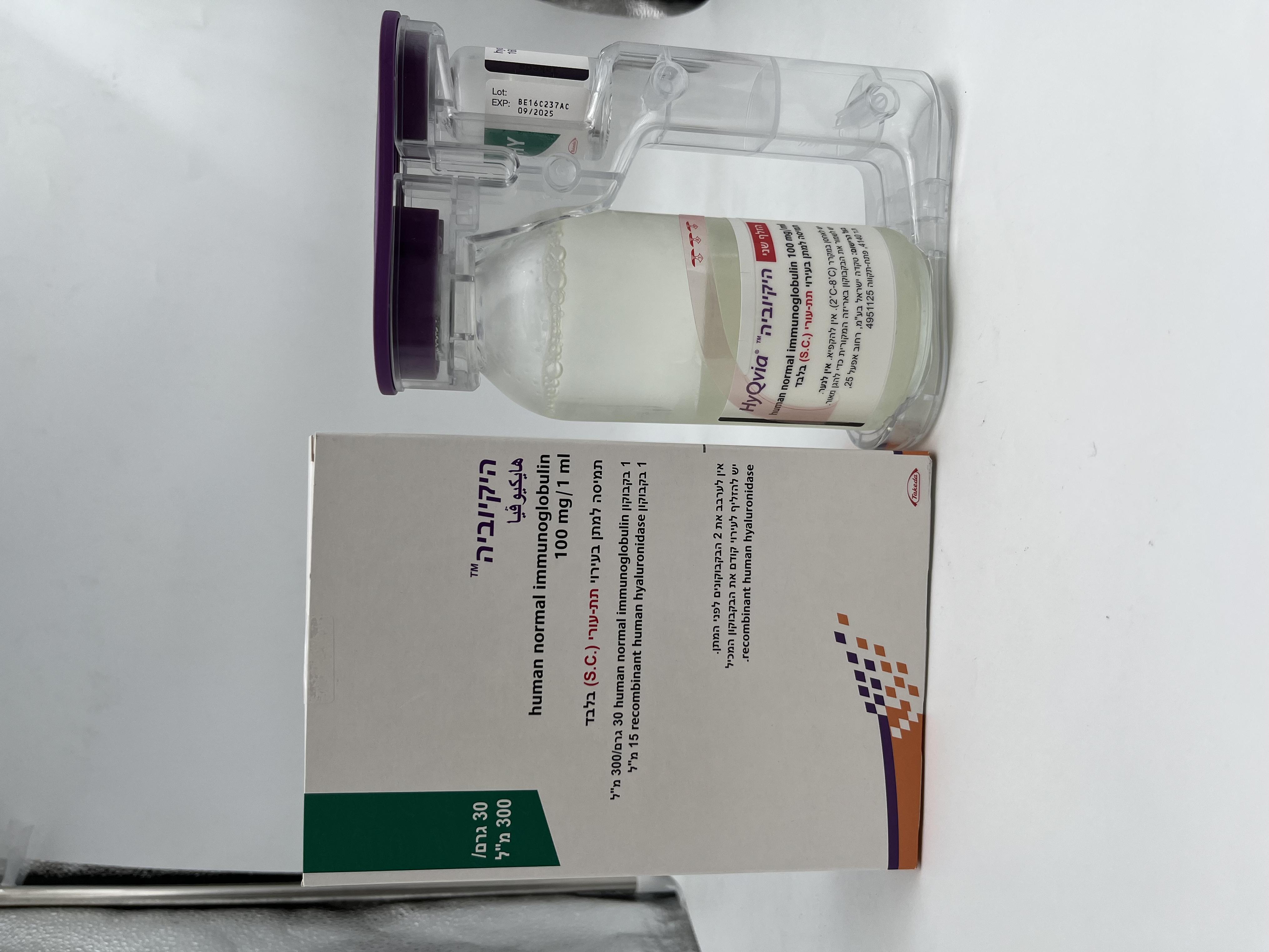
היקיוביה HYQVIA (HUMAN NORMAL IMMUNOGLOBULIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה לאינפוזיה : SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Traceability In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Precautions for use If HyQvia is accidentally administered into a blood vessel, patients could develop shock. The recommended infusion rate given in section 4.2 should be adhered to. Patients must be closely monitored throughout the infusion period, particularly patients starting with therapy. Certain adverse reactions may occur more frequently in patients who receive human normal immunoglobulin for the first time or, in rare cases, when the human normal immunoglobulin product is switched or when there has been a long interval since the previous infusion. Potential complications can often be avoided by: • initially infusing the product slowly (see section 4.2). • ensuring that patients are carefully monitored for any symptoms throughout the infusion period. In particular, patients naive to human normal immunoglobulin, patients switched from an alternative immunoglobulin product or when there has been a long interval since the previous infusion should be monitored during the first infusion and for the first hour after the first infusion, to detect potential adverse signs. All other patients should be observed for at least 20 minutes after the administration. When treatment is given at home, support from another responsible person should be available for treating adverse reactions or to summon help should a serious adverse reaction occur. Patients on self- home treatment and/or their guardian should also be trained to detect early signs of hypersensitivity reactions. In case of adverse reaction, either the rate of administration must be reduced, or the infusion stopped. The treatment required depends on the nature and severity of the adverse reaction. In case of shock, immediately discontinue the infusion and treat the patient for shock. No chronic changes in the skin were observed in the clinical studies. Patients should be reminded to report any chronic inflammation, nodules or inflammation that occurs at the infusion site and lasts more than a few days. Hypersensitivity to IG 10% True hypersensitivity reactions are rare. They can particularly occur in patients with anti-IgA antibodies who should be treated with particular caution. Patients with anti-IgA antibodies, in whom treatment with SCIg products remains the only option, should be treated with HyQvia only under close medical supervision. Rarely, human normal immunoglobulin can induce a fall in blood pressure with anaphylactic reaction, even in patients who had tolerated previous treatment with human normal immunoglobulin. • If a patient is at high risk for any allergic reactions, the medicinal product should be administered only where supportive care is available for life threatening reactions. • Patients should be informed of the early signs of anaphylaxis/hypersensitivity (hives, pruritus, generalized urticaria, tightness of the chest, wheezing, and hypotension). • Depending on the severity of associated reaction, and medical practice, pre-medication may prevent this type of reaction. • If known anaphylactic or severe hypersensitivity to human immunoglobulin exists, it should be noted in the patient records. Hypersensitivity to rHuPH20 Any suspicion of allergic or anaphylactic like reactions following rHuPH20 administration requires immediate discontinuation of the infusion and standard medical treatment should be administered, if necessary. Immunogenicity of rHuPH20 Development of non-neutralizing antibodies and neutralizing antibodies to the rHuPH20 component has been reported in patients receiving HyQvia in clinical studies. The potential exists for such antibodies to cross-react with endogenous hyaluronidase, which is known to be expressed in the adult male testes, epididymis, and sperm. It is unknown whether these antibodies may have any clinical significance in humans (see section 4.8). Thromboembolism Arterial and venous thromboembolic events including myocardial infarction, stroke, deep venous thrombosis and pulmonary embolism have been associated with the use of immunoglobulins. Patients should be sufficiently hydrated before using immunoglobulins. Caution should be exercised in patients with pre-existing risk factors for thromboembolic events (such as advanced age, hypertension, diabetes mellitus and a history of vascular disease or thrombotic episodes, patients with acquired or inherited thrombophilic disorders, patients with prolonged periods of immobilization, severely hypovolaemic patients, patients with diseases which increase blood viscosity). Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. Thrombosis may also occur in the absence of known risk factors. Patients should be informed about first symptoms of thromboembolic events including shortness of breath, pain and swelling of a limb, focal neurological deficits and chest pain and should be advised to contact their physician immediately upon onset of symptoms. Haemolytic anaemia Immunoglobulin products contain antibodies to blood groups (e.g. A, B, D) which may act as haemolysins. These antibodies bind to red blood cells (RBC) epitopes (which may be detected as a positive direct antiglobulin test [DAT, (Coombs’ test)] and, rarely, may cause haemolysis. Immunoglobulin product recipients should be monitored for clinical signs and symptoms of haemolysis. Aseptic meningitis syndrome (AMS) Aseptic meningitis syndrome has been reported to occur in association with IVIg and SCIg treatment; the symptoms usually begin within several hours to 2 days following immunoglobulin treatment. Patients should be informed about the first symptoms which encompass severe headache, neck stiffness, drowsiness, fever, photophobia, nausea, and vomiting. Discontinuation of immunoglobulin treatment may result in remission of AMS within several days without sequelae. Cerebrospinal fluid studies are frequently positive with pleocytosis up to several thousand cells per mm3, predominantly from the granulocytic series, and elevated protein levels up to several hundred mg/dL. AMS may occur more frequently in association with high-dose (2 g/kg) IVIg treatment. From post-marketing data no clear correlation of AMS to higher doses was observed. Higher incidences of AMS were seen in women. Interference with serological testing After infusion of immunoglobulins, the transitory rise of the various passively transferred antibodies in the patient’s blood may result in misleading positive results in serological testing. Passive transmission of antibodies to erythrocyte´s surface antigens, (e.g., A, B, D) may interfere with some serological tests for red cell antibodies for example the direct antiglobulin test (DAT, direct Coombs’ test). Infusions of immunoglobulin products may lead to false positive readings in assays that depend on detection of β-D-glucans for diagnosis of fungal infections; this may persist during the weeks following infusion of the product. Transmissible agents Human normal immunoglobulin and human serum albumin (stabilizer of the rHuPH20) are produced from human plasma. Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infectious agents cannot be totally excluded. This also applies to unknown or emerging viruses and other pathogens. The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV), and for the non-enveloped hepatitis A (HAV) and parvovirus B19 viruses. There is reassuring clinical evidence regarding the lack of hepatitis A or parvovirus B19 transmission with immunoglobulins and it is also assumed that the antibody content makes an important contribution to viral safety. Sodium content The IG 10% component is essentially sodium-free. The rHuPH20 contains the following amount (mg) of sodium per vial: 1.25 mL contains 5.0 mg of sodium. 2.5 mL contains 10.1 mg of sodium. 5 mL contains 20.2 mg of sodium. 10 mL contains 40.3 mg of sodium. 15 mL contains 60.5 mg of sodium. This is equivalent to 0.25 to 3% of the WHO recommended maximum daily intake of 2 g sodium for an adult. Paediatric population The listed warnings and precautions apply both to adults and children.
Effects on Driving
4.7 Effects on ability to drive and use machines The ability to drive and operate machines may be impaired by some adverse reactions e.g., dizziness (see section 4.8) associated with this medicinal product. Patients who experience adverse reactions during treatment should wait for these to resolve before driving or operating machines.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול במקרים האלה: א. חסר חיסוני ראשוני (חולים עם פגיעה ראשונית בייצור נוגדנים כגון אגמגלובולינמיה או היפוגמגלובוילינמיה, ITP (Idiopathic thrombocytopenic purpura)); ב. חסר חיסוני ספציפי, מניעה או טיפול בחצבת, הפטיטיס A ויראלית; ג. CIDP – Chronic inflammatory demyelineating polyneuropathy; ד.טיפול בחולי לוקמיה מסוג CLL הסובלים מהיפוגלמגלובולינמיה משנית חמורה וזיהומים חוזרים.
שימוש לפי פנקס קופ''ח כללית 1994
Primary immunodeficiency (patients with primary defective antibody synthesis such as agammaglobulinemia or hypogammaglobulinemia, idiopathic thrombocytopenic purpura (ITP)
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
עלון מידע לצרכן
18.11.21 - עלון לצרכן אנגלית 18.11.21 - עלון לצרכן עברית 18.11.21 - עלון לצרכן אנגלית 18.11.21 - עלון לצרכן עברית 15.06.21 - עלון לצרכן אנגלית 15.07.21 - עלון לצרכן עברית 15.06.21 - עלון לצרכן ערבית 06.11.22 - עלון לצרכן אנגלית 06.11.22 - עלון לצרכן עברית 06.11.22 - עלון לצרכן ערבית 12.10.23 - עלון לצרכן אנגלית 12.10.23 - עלון לצרכן עברית 12.10.23 - עלון לצרכן ערבית 14.09.22 - החמרה לעלוןלתרופה במאגר משרד הבריאות
היקיוביה