Quest for the right Drug
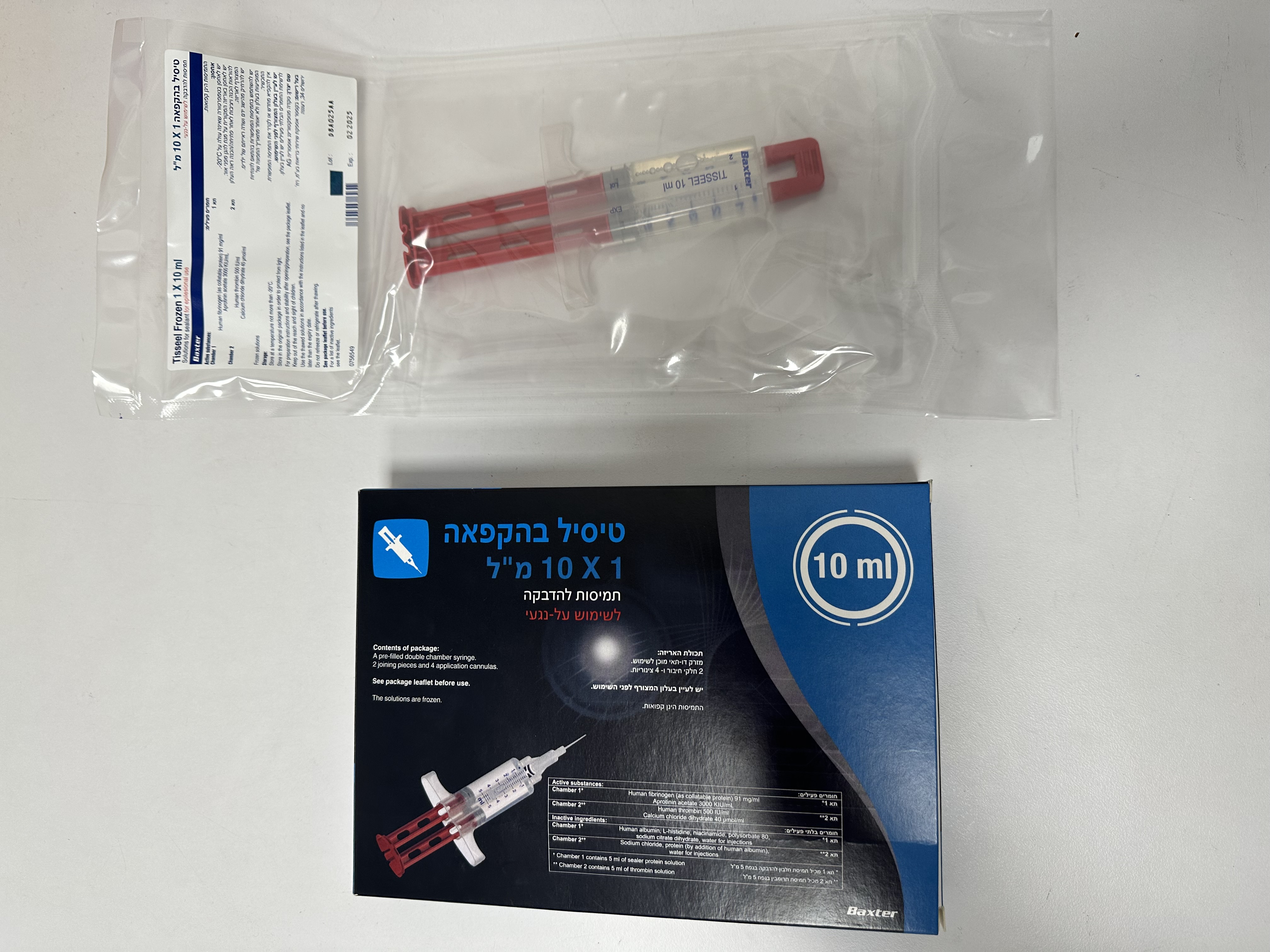
טיסיל בהקפאה TISSEEL FROZEN (APROTININ ACETATE, CALCIUM CHLORIDE DIHYDRATE, HUMAN FIBRINOGEN, HUMAN THROMBIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
לסביבת הפצע : EPILESIONAL
צורת מינון:
אין פרטים : SOLUTIONS FOR SEALANT
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use For epilesional use only. Do not apply intravascularly. Life threatening thromboembolic complications may occur if the preparation is unintentionally applied intravascularly. Caution must be used when applying fibrin sealant using pressurized gas. Any application of pressurized gas is associated with a potential risk of air or gas embolism, tissue rupture, or gas entrapment with compression, which may be life-threatening. Apply TISSEEL FROZEN as a thin layer. Excessive clot thickness may negatively interfere with the product’s efficacy and the wound healing process. Life-threatening/fatal air or gas embolism has occurred with the use of spray devices employing a pressure regulator to administer fibrin sealants. This event appears to be related to the use of the spray device at higher than recommended pressures and/or in close proximity to the tissue surface. The risk appears to be higher when fibrin sealants are sprayed with air, as compared to CO2 and therefore cannot be excluded with TISSEEL FROZEN when sprayed in open wound surgery. When applying TISSEEL FROZEN using a spray device, be sure to use a pressure within the pressure range recommended by the spray device manufacturer (see table in section 6.6 for pressures and distances). TISSEEL FROZEN spray application should only be used if it is possible to accurately judge the spray distance as recommended by the manufacturer. Do not spray closer than the recommended distances. When spraying TISSEEL FROZEN, changes in blood pressure, pulse, oxygen saturation and end tidal CO2 should be monitored because of the possibility of occurrence of air or gas embolism (also see section 4.2). TISSEEL FROZEN must not be used with the Easy Spray / Spray Set system in enclosed body areas. Before the administration of TISSEEL FROZEN, care is to be taken that parts of the body outside the designated application area are sufficiently protected/covered to prevent tissue adhesion at undesired sites. If fibrin sealants are applied in confined spaces, e.g. the brain or the spinal cord, the risk of compressive complications should be taken into account. To ensure adequate mixing of the sealer protein component and the thrombin component, the first few drops of the product from the application cannula should be expelled and discarded immediately before use. As with any protein-containing product, allergic type hypersensitivity reactions are possible. Intravascular application might increase the likelihood and severity of acute hypersensitivity reactions in susceptible patients. Hypersensitivity and anaphylactic reactions (also fatal, including anaphylactic shock) have been reported with TISSEEL FROZEN. Signs of hypersensitivity reactions may include hives, generalized urticaria, tightness of the chest, wheezing, hypotension. If these symptoms occur, the administration must be discontinued immediately and the currently valid standard measures for the treatment of shock are to be taken. Remaining product must be removed from the site of application. TISSEEL FROZEN contains a synthetic protein (aprotinin). Even in case of strict local application, there is a risk of anaphylactic reaction linked to the presence of aprotinin. The risk seems to be higher in cases where there was previous exposure, even if it was well tolerated. Therefore, any use of aprotinin or aprotinin containing products should be recorded in the patients’ records. As synthetic aprotinin is structurally identical to bovine aprotinin the use of TISSEEL FROZEN in patients with allergies to bovine proteins should be carefully evaluated. In two retrospective, non-randomized studies in coronary bypass surgery, patients who received fibrin sealant showed a statistically significant increased risk of mortality. While these studies could not provide a causal relationship, the increased risk associated with the use of TISSEEL FROZEN in these patients cannot be excluded. Therefore, additional care should be taken to avoid inadvertent intravascular administration of this product. Injection into the nasal mucosa must be avoided, as thromboembolic complications may occur in the area of the arteria ophthalmica. Injecting TISSEEL FROZEN into tissue carries the risk of local tissue damage. TISSEEL FROZEN should only be applied as a thin layer. Excessive clot thickness may negatively interfere with the product’s efficacy and the wound healing process. Polysorbate 80 may cause locally limited skin irritations such as contact dermatitis. Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses and other pathogens. These measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), and for the non-enveloped hepatitis A virus (HAV). The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals with immunodeficiency or increased erythropoiesis (e.g., hemolytic anemia). Appropriate vaccination (hepatitis A and B) should be considered for patients in regular/repeated receipt of human plasma-derived fibrin sealant. It is strongly recommended that every time that TISSEEL FROZEN is administered to the patient, the name and batch number of the product are recorded in order to maintain a link between the patient and the batch of the product. Oxidized cellulose-containing preparations should not be used with TISSEEL FROZEN (See section 6.2 Incompatibilities).
Effects on Driving
4.7 Effects on ability to drive and use machines Not relevant.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
רישום
162 87 35322 00
מחיר
0 ₪
מידע נוסף
