Quest for the right Drug
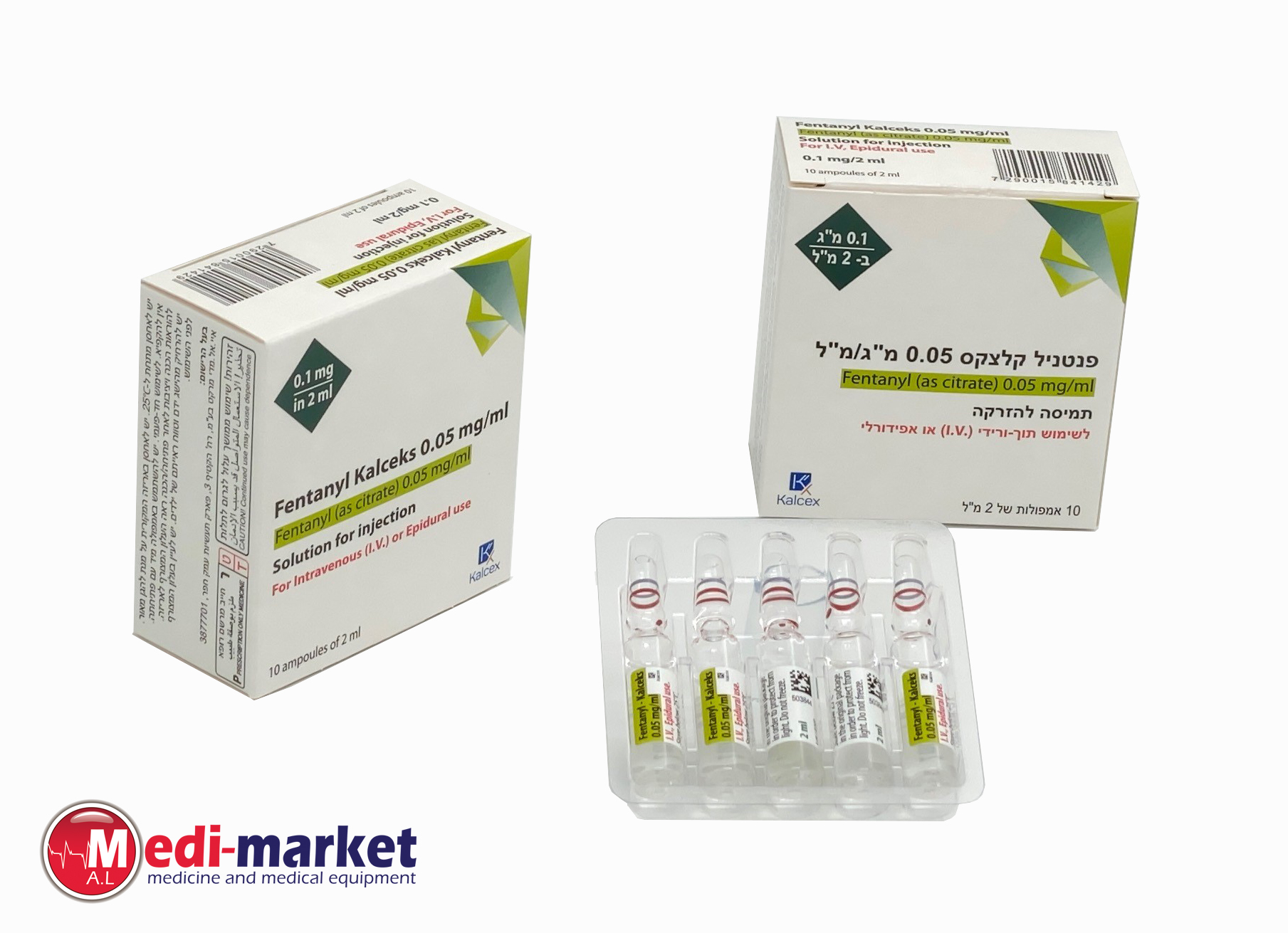
פנטניל - קלצקס 0.05 מ"ג/מ"ל FENTANYL - KALCEKS 0.05 MG/ML (FENTANYL AS CITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
אפידורל, תוך-ורידי : EPIDURAL, I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6 PHARMACEUTICAL PARTICULARS 6.1 List of excipients Sodium chloride Sodium hydroxide (for pH adjustment) Water for injections 6.2 Incompatibilities The product is chemically incompatible with the induction agents thiopentone and methohexitone because of the wide differences in pH. This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.3. 6.3 Shelf life The expiry date of the product is indicated on the package materials. After dilution: Fentanyl-Kalceks 0.05 mg/ml solution for injection is stable for 72 h in 5% glucose solution and 0.9% sodium chloride solution at ambient temperature. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user. Dilution should take place in controlled and validated aseptic conditions. 6.4 Special precautions for storage Do not store above 25 °C. Store in the original package in order to protect from light. Do not freeze. 6.5 Nature and contents of container 10 glass ampoules of 2 ml 10 glass ampoules of 10 ml Not all pack sizes may be marketed. 6.6 Special precautions for disposal and other handling For single use only. If only part used, discard the remaining solution. Use finger protection when opening an ampoule. Instruction of ampoule opening: 1) Turn the ampoule with coloured point up. If there is any solution in the upper part of the ampoule, gently tap with your finger to get all the solution to the lower part of the ampoule. 2) Use both hands to open; while holding the lower part of the ampoule in one hand, use the other hand to break off the upper part of the ampoule in the direction away from the coloured point (see the pictures below). Any unused medicinal product or waste material should be disposed of in accordance with local requirements. Accidental dermal exposure should be treated by rinsing the affected area with water. Avoid usage of soap, alcohol, and other cleaning materials that may cause chemical or physical abrasions to the skin. Store as a CD.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
