Quest for the right Drug
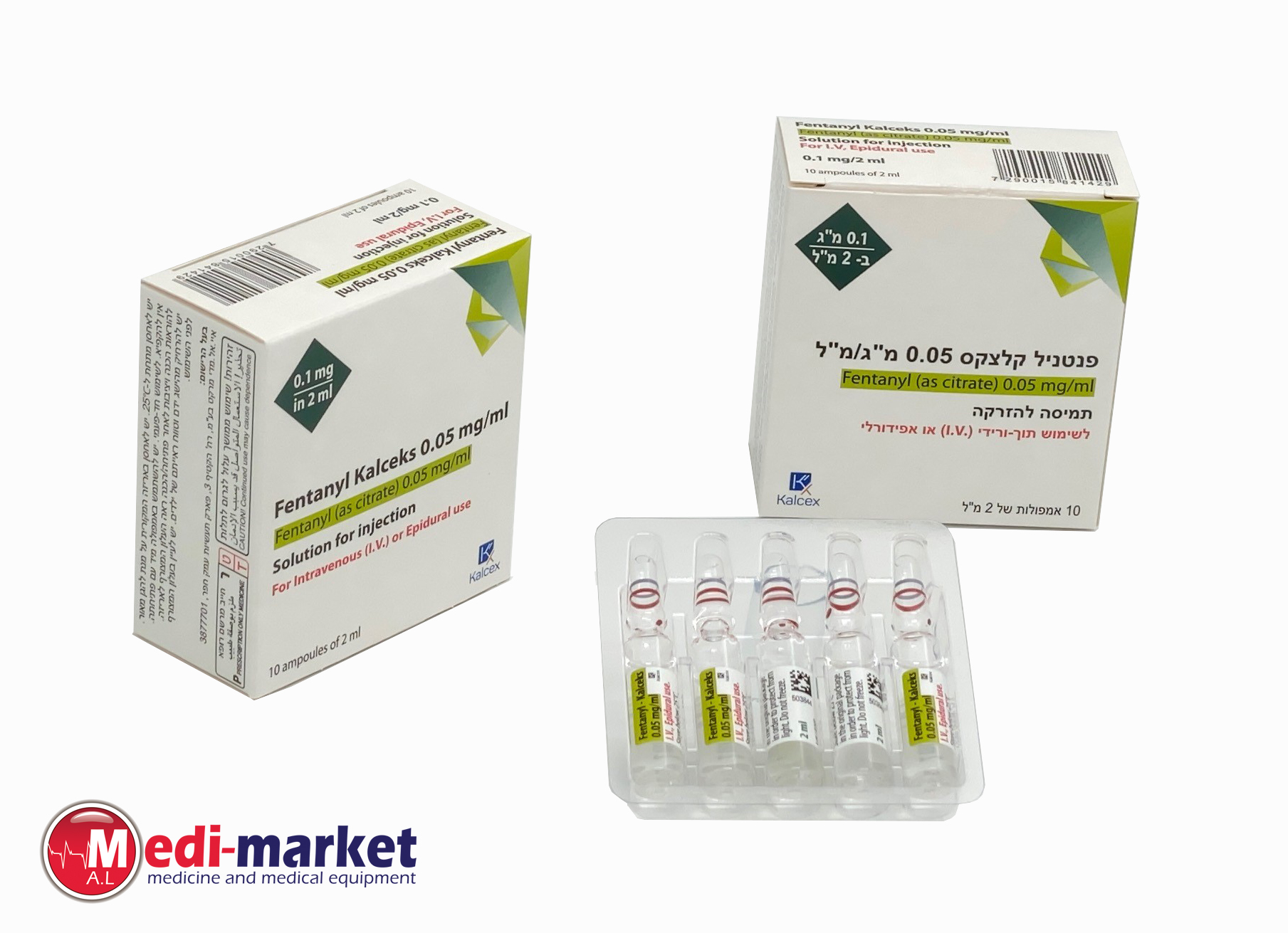
פנטניל - קלצקס 0.05 מ"ג/מ"ל FENTANYL - KALCEKS 0.05 MG/ML (FENTANYL AS CITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
אפידורל, תוך-ורידי : EPIDURAL, I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pregnancy & Lactation : הריון/הנקה
4.6 Fertility, pregnancy and lactation Pregnancy Fentanyl can cross the placenta in early pregnancy. Studies in animals have shown some reproductive toxicity (see Section 5.3, Preclinical safety data). Administration during childbirth (including Caesarean section) is not recommended because fentanyl crosses the placenta and may suppress spontaneous respiration in the new-born period. If fentanyl is administered, assisted ventilation equipment must be immediately available for the mother and infant if required. An opioid antagonist for the child must always be available. Regular use during pregnancy may cause drug dependence in the foetus, leading to withdrawal symptoms in the neonate. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. Administration during labour may depress respiration in the neonate and an antidote for the child should be readily available. Breast-feeding Administration to nursing women is not recommended as fentanyl may be secreted in breast milk and may cause respiratory depression in the infant. The risk/benefit of breast-feeding following fentanyl administration should be considered. Fertility There are no clinical data on the effects of fentanyl on male or female fertility. In animal studies, some tests on rats showed reduced female fertility at maternal toxic doses (see section 5.3 Preclinical safety data).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
