Quest for the right Drug
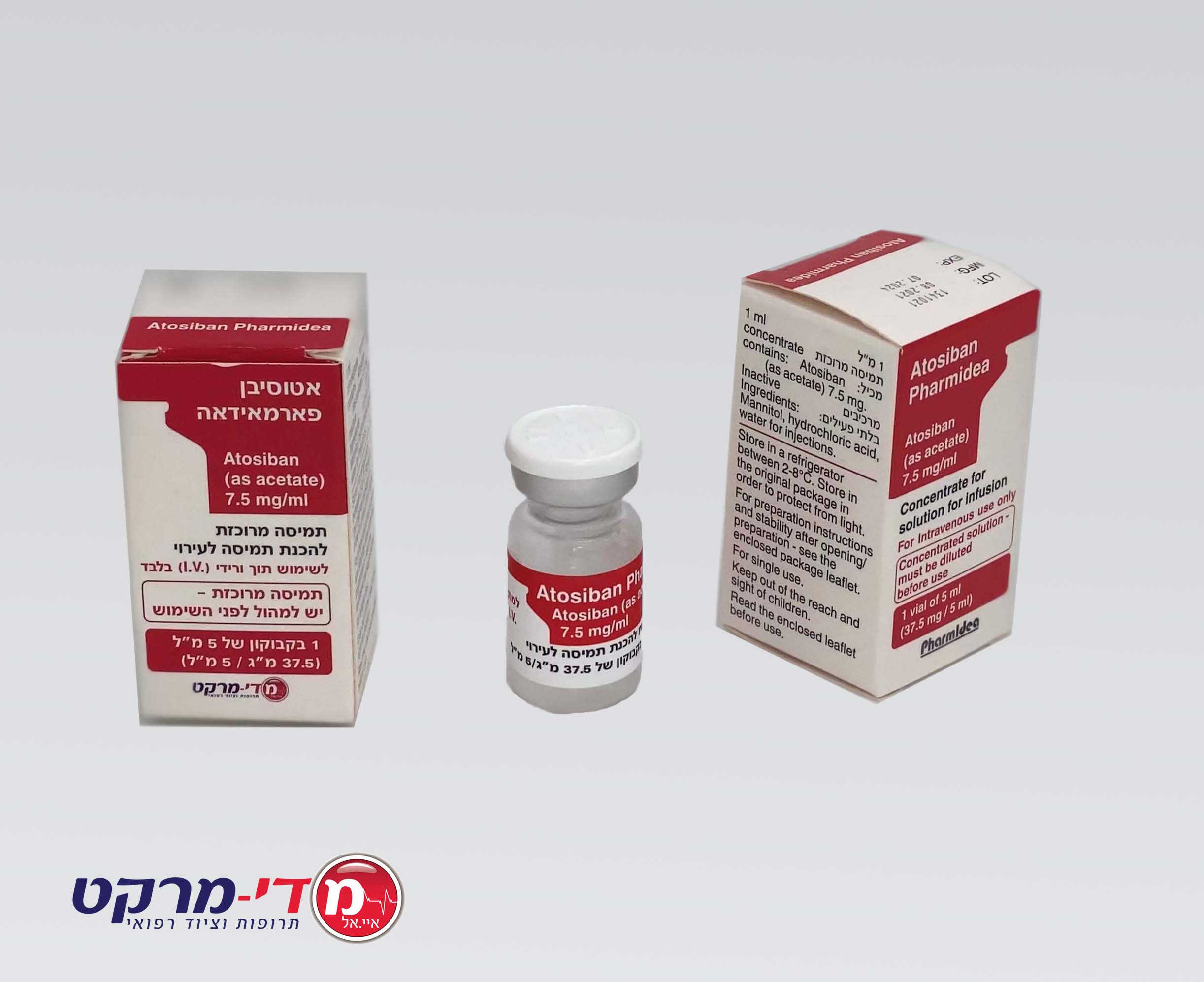
אטוסיבן פארמאידאה ATOSIBAN PHARMIDEA (ATOSIBAN AS ACETATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אין פרטים : SOLUTION FOR INJECTION/ CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1. Pharmacodynamic properties Pharmacotherapeutic group: Other gynecologicals, ATC code: G02CX01 Atosiban Pharmidea contains atosiban (INN), a synthetic peptide ([Mpa1,D-Tyr(Et)2,Thr4,Orn8]-oxytocin) which is a competitive antagonist of human oxytocin at receptor level. In rats and guinea pigs, atosiban was shown to bind to oxytocin receptors, to decrease the frequency of contractions and the tone of the uterine musculature, resulting in a suppression of uterine contractions. Atosiban was also shown to bind to the vasopressin receptor, thus inhibiting the effect of vasopressin. In animals, atosiban did not exhibit cardiovascular effects. In human pre-term labour, atosiban at the recommended dosage antagonises uterine contractions and induces uterine quiescence. The onset of uterus relaxation following atosiban is rapid, uterine contractions being significantly reduced within 10 minutes to achieve stable uterine quiescence (≤4 contractions/hour) for 12 hours. Phase III clinical trials (CAP-001 studies) include data from 742 women who were diagnosed with pre-term labour at 23–33 weeks of gestation and were randomised to receive either atosiban (according to this labelling) or β-agonist (dose-titrated). Primary endpoint: The primary efficacy outcome was the proportion of women remaining undelivered and not requiring alternative tocolysis within 7 days of treatment initiation. The data show that 59.6% (n=201) and 47.7% (n=163) of atosiban- and β-agonist-treated women (p=0.0004), respectively, were undelivered and did not require alternative tocolysis within 7 days of starting treatment. Most of the treatment failures in CAP-001 were caused by poor tolerability. Treatment failures caused by insufficient efficacy were significantly (p=0.0003) more frequent in atosiban (n=48, 14.2%) than in the β-agonist- treated women (n=20, 5.8%). In the CAP-001 studies the probability of remaining undelivered and not requiring alternative tocolytics within 7 days of treatment initiation was similar for atosiban and beta-mimetics treated women at gestational age of 24-28 weeks. However, this finding is based on a very small sample (n=129 patients). Secondary endpoints: Secondary efficacy parameters included the proportion of women remaining undelivered within 48 h of treatment initiation. There was no difference between the atosiban and beta-mimetic groups with regard to this parameter. Mean (SD) gestational age at delivery was the same in the two groups: 35.6 (3.9) and 35.3 (4.2) weeks for the atosiban and β-agonist groups, respectively (p=0.37). Admission to a neonatal intensive care unit (NICU) was similar for both treatment groups (approximately 30%), as was length of stay and ventilation therapy. Mean (SD) birth weight was 2491 (813) g in the atosiban group and 2461 (831) g in the β- agonist group (p=0.58). Foetal and maternal outcome did apparently not differ between the atosiban and the β-agonist group, but the clinical studies were not powered enough to rule out a possible difference. Of the 361 women who received atosiban treatment in the phase III studies, 73 received at least one re-treatment, 8 received at least 2 re- treatments and 2 received 3 re-treatments (see section 4.4). As the safety and efficacy of atosiban in women with a gestational age of less than 24 completed weeks has not been established in controlled randomized studies, the treatment of this patient group with atosiban is not recommended (see section 4.3). In a placebo-controlled study, foetal/infant deaths were 5/295 (1.7%) in the placebo group and 15/288 (5.2%) in the atosiban group, of which two occurred at five and eight months of age. Eleven out of the 15 deaths in the atosiban group occurred in pregnancies with a gestational age of 20 to 24 weeks, although in this subgroup patient distribution was unequal (19 women on atosiban, 4 on placebo). For women with a gestational age greater than 24 weeks there was no difference in mortality rate (1.7% in the placebo group and 1.5% in the atosiban group).
Pharmacokinetic Properties
5.2. Pharmacokinetic properties In healthy non-pregnant subjects receiving atosiban infusions (10 to 300 micrograms/min over 12 hours), the steady state plasma concentrations increased proportionally to the dose. The clearance, volume of distribution and half-life were found to be independent of the dose. In women in pre-term labour receiving atosiban by infusion (300 micrograms/min for 6 to 12 hours), steady state plasma concentrations were reached within one hour following the start of the infusion (mean 442 ± 73 ng/ml, range 298 to 533 ng/ml). Following completion of the infusion, plasma concentration rapidly declined with an initial (tα) and terminal (tβ) half-life of 0.21 ± 0.01 and 1.7 ± 0.3 hours, respectively. Mean value for clearance was 41.8 ± 8.2 l/h. Mean value of volume of distribution was 18.3 ± 6.8 l. Plasma protein binding of atosiban is 46 to 48% in pregnant women. It is not known whether the free fraction in the maternal and foetal compartments differs substantially. Atosiban does not partition into red blood cells. Atosiban passes the placenta. Following an infusion of 300 µg/min in healthy pregnant women at term, the foetal/maternal atosiban concentration ratio was 0.12. Two metabolites were identified in the plasma and urine from human subjects. The ratios of the main metabolite M1 (des-(Orn8, Gly-NH₂9)- [Mpa1, D-Tyr(Et)2, Thr4]-oxytocin) to atosiban concentrations in plasma were 1.4 and 2.8 at the second hour and at the end of the infusion respectively. It is not known whether M1 accumulates in tissues. Atosiban is found in only small quantities in urine, its urinary concentration is about 50 times lower than that of M1. The proportion of atosiban eliminated in faeces is not known. The main metabolite M1 is approximately 10 times less potent than atosiban in inhibiting oxytocin-induced uterine contractions in vitro. Metabolite M1 is excreted in milk (see section 4.6). There is no experience with atosiban treatment in patients with impaired function of the liver or kidneys. Renal impairment is not likely to warrant a dose adjustment, since only a small extent of atosiban is excreted in the urine. In patients with impaired hepatic function, atosiban should be used with caution (see sections 4.2 and 4.4). It is unlikely that atosiban inhibits hepatic cytochrome P₄₅₀ isoforms in humans (see section 4.5).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
