Quest for the right Drug
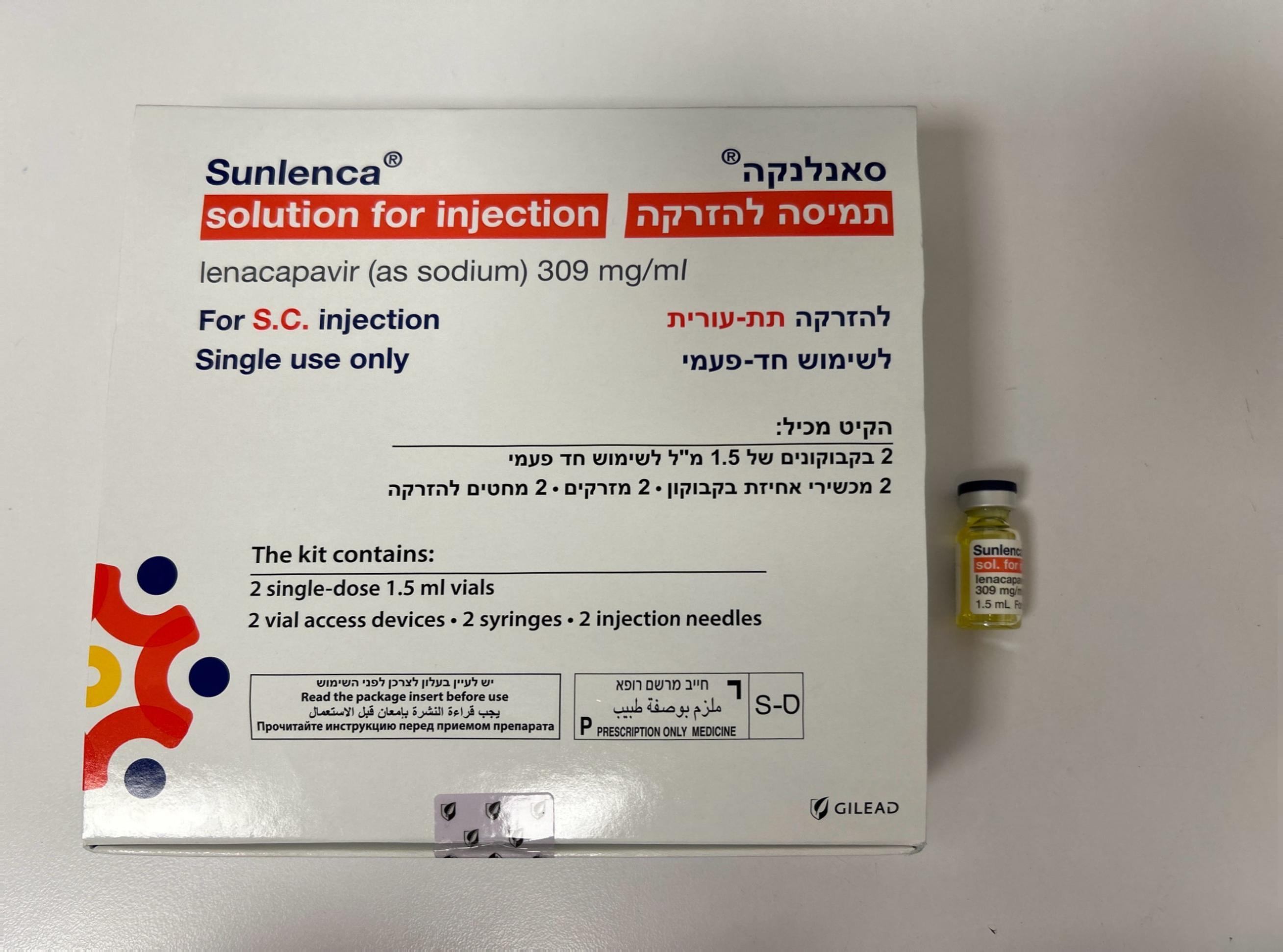
סאנלנקה תמיסה להזרקה SUNLENCA SOLUTION FOR INECTION (LENACAPAVIR AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Interactions : אינטראקציות
4.5 Interaction with other medicinal products and other forms of interaction Effect of other medicinal products on the pharmacokinetics of lenacapavir Lenacapavir is a substrate of CYP3A, P-gp and UGT1A1. Strong inducers of CYP3A, P-gp, and UGT1A1, such as rifampicin, may significantly decrease plasma concentrations of lenacapavir resulting in loss of therapeutic effect and development of resistance, therefore co-administration is contraindicated (see section 4.3). Moderate inducers of CYP3A and P-gp, such as efavirenz, may also significantly decrease plasma concentrations of lenacapavir, therefore co-administration is not recommended (see section 4.4). Strong inhibitors of CYP3A, P-gp and UGT1A1 together (i.e., all 3 pathways), such as atazanavir/cobicistat, may significantly increase plasma concentrations of lenacapavir, therefore co-administration is not recommended (see section 4.4). Strong CYP3A4 inhibitors alone (e.g. voriconazole) or strong inhibitors of CYP3A4 and P-gp together (e.g. cobicistat) do not result in a clinically meaningful increase in lenacapavir exposures. Effect of lenacapavir on the pharmacokinetics of other medicinal products Lenacapavir is a moderate inhibitor of CYP3A and a P-gp inhibitor. Caution is advised if Sunlenca is co-administered with a sensitive CYP3A and/or P-gp substrate with a narrow therapeutic index. Lenacapavir is not a clinically meaningful inhibitor of BCRP and does not inhibit OATP. Table 2: Interactions between Sunlenca and other medicinal products Medicinal product by Effects on concentrations. Recommendation concerning therapeutic areas Mean percent change in AUC, co-administration with Cmax Sunlenca ANTIMYCOBACTERIALS Rifampicina,b,c (600 mg once daily) Lenacapavir: Co-administration is AUC: ↓84% contraindicated (see section 4.3). Cmax: ↓55% Rifabutin Interaction not studied. Co-administration is not recommended (see section 4.4). Co-administration of rifabutin may decrease lenacapavir plasma concentrations, which may result in loss of therapeutic effect and development of resistance. ANTICONVULSANTS Carbamazepine Interaction not studied. Co-administration is Phenytoin contraindicated (see section 4.3). Oxcarbazepine Co-administration of Co-administration is not Phenobarbital carbamazepine, oxcarbazepine, recommended (see section 4.4). phenobarbital, or phenytoin with lenacapavir may decrease Alternative anticonvulsants lenacapavir plasma concentrations, should be considered. which may result in loss of therapeutic effect and development of resistance. Medicinal product by Effects on concentrations. Recommendation concerning therapeutic areas Mean percent change in AUC, co-administration with Cmax Sunlenca HERBAL PRODUCTS St. John’s wort (Hypericum Interaction not studied. Co-administration is perforatum) contraindicated (see section 4.3). Co-administration of St. John’s wort may decrease lenacapavir plasma concentrations, which may result in loss of therapeutic effect and development of resistance. ANTIRETROVIRAL AGENTS Atazanavir/cobicistatb,d,e Lenacapavir: Co-administration is not (300 mg/150 mg once daily) AUC: ↑ 321% recommended (see section 4.4). Cmax: ↑ 560% Efavirenzb,d,f (600 mg once daily) Lenacapavir: AUC:↓ 56% Cmax:↓ 36% Etravirine Interaction not studied. Nevirapine Tipranavir/ritonavir Co-administration of etravirine, nevirapine, or tipranavir/ritonavir may decrease lenacapavir plasma concentrations, which may result in loss of therapeutic effect and development of resistance. Cobicistatb,d,g (150 mg once daily) Lenacapavir: No dose adjustment of AUC: ↑ 128% lenacapavir is required. Cmax:↑ 110% Darunavir/cobicistatb,d,h Lenacapavir: (800 mg/150 mg once daily) AUC:↑ 94% Cmax:↑ 130% Ritonavir Interaction not studied. Co-administation of ritonavir may increase lenacapavir plasma concentrations. Tenofovir alafenamided,i,j (25 mg) Tenofovir alafenamide: No dose adjustment of tenofovir AUC:↑ 32% alafenamide is required. Cmax:↑ 24% Tenofovirk: AUC:↑ 47% Cmax:↑ 23% ERGOT DERIVATIVES Dihydroergotamine Interaction not studied. Caution is warranted when Ergotamine dihydroergotamine or ergotamine, Plasma concentrations of these is co-administered with Sunlenca. medicinal products may be increased when co-administered with lenacapavir. PHOSPHODIESTERASE-5 (PDE-5) INHIBITORS Sildenafil Interaction not studied. Use of PDE-5 inhibitors for Tadalafil pulmonary arterial hypertension: Vardenafil Plasma concentration of PDE-5 Co-administration with tadalafil is inhibitors may be increased when not recommended. co-administered with lenacapavir. Use of PDE-5 inhibitors for erectile dysfunction: Sildenafil: A starting dose of 25 mg is recommended. Medicinal product by Effects on concentrations. Recommendation concerning therapeutic areas Mean percent change in AUC, co-administration with Cmax Sunlenca Vardenafil: No more than 5 mg in a 24-hour period. Tadalafil: • For use as needed: no more than 10 mg every 72 hours • For once daily use: dose not to exceed 2.5 mg CORTICOSTEROIDS (systemic) Dexamethasone Interaction not studied. Co-administration of Sunlenca Hydrocortisone/cortisone with corticosteroids whose Plasma concentrations of exposures are significantly corticosteroids may be increased increased by CYP3A inhibitors when co-administered with can increase the risk for Cushing's lenacapavir. syndrome and adrenal suppression. Initiate with the lowest starting dose and titrate Plasma concentrations of carefully while monitoring for lenacapavir may decrease when safety. co-administered with systemic dexamethasone, which may result Caution is warranted when in loss of therapeutic effect and systemic dexamethasone is development of resistance. co-administered with Sunlenca, particularly for long-term use. Alternative corticosteroids should be considered. HMG-CoA REDUCTASE INHIBITORS Lovastatin Interaction not studied. Initiate lovastatin and simvastatin Simvastatin with the lowest starting dose and Plasma concentrations of these titrate carefully while monitoring medicinal products may be for safety (e.g. myopathy). Atorvastatin increased when co-administered No dose adjustment of with lenacapavir. atorvastatin is required. Pitavastatind,i,l (2 mg single dose; Pitavastatin: No dose adjustment of simultaneous or 3 days after AUC:↔ pitavastatin and rosuvastatin is lenacapavir) Cmax:↔ required. Rosuvastatind,i,m (5 mg single Rosuvastatin: dose) AUC:↑ 31% Cmax:↑ 57% ANTIARRHYTHMICS Digoxin Interaction not studied. Caution is warranted and therapeutic concentration Plasma concentration of digoxin monitoring of digoxin is may be increased when recommended. co-administered with lenacapavir. Medicinal product by Effects on concentrations. Recommendation concerning therapeutic areas Mean percent change in AUC, co-administration with Cmax Sunlenca SEDATIVES/HYPNOTICS Midazolamd,i,n (2.5 mg single dose; Midazolam: Caution is warranted when oral; simultaneous administration) AUC: ↑ 259% midazolam or triazolam, is Cmax: ↑ 94% co-administered with Sunlenca. 1-hydroxymidazolamo: AUC: ↓ 24% Cmax: ↓ 46% Midazolamd,i,n (2.5 mg single Midazolam: dose; oral;1 day after lenacapavir) AUC: ↑ 308% Cmax: ↑ 116% 1-hydroxymidazolamo: AUC: ↓ 16% Cmax: ↓ 48% Triazolam Interaction not studied. Plasma concentration of triazolam may be increased when co-administered with lenacapavir. ANTICOAGULANTS Direct Oral Anticoagulants Interaction not studied. Due to potential bleeding risk, (DOACs) dose adjustment of DOAC may be Rivaroxaban Plasma concentration of DOAC required. Consult the Summary of Dabigatran may be increased when Product Characteristics of the Edoxaban co-administered with lenacapavir. DOAC for further information on use in combination with moderate CYP3A inhibitor and/or P-gp inhibitors. ANTIFUNGALS Voriconazolea,b,p,q (400 mg twice Lenacapavir: No dose adjustment of daily/200 mg twice daily) AUC:↑ 41% lenacapavir is required. Cmax:↔ Itraconazole Interaction not studied. Ketoconazole Plasma concentration of lenacapavir may be increased when co-administered with itraconazole or ketoconazole. H2-RECEPTOR ANTAGONISTS Famotidinea,b (40 mg once daily, 2 Famotidine: No dose adjustment of famotidine hours before lenacapavir) AUC:↑ 28% is required. Cmax:↔ ORAL CONTRACEPTIVES Ethinylestradiol Interaction not studied. No dose adjustment of Progestins ethinylestradiol and progestins is Plasma concentrations of required. ethinylestradiol and progestins may be increased when co-administered with lenacapavir. GENDER AFFIRMING HORMONES 17β-estradiol Interaction not studied. No dose adjustment of these Anti-androgens gender affirming hormones is Progestogen Plasma concentrations of these required. Testosterone medicinal products may be increased when co-administered with lenacapavir. a Fasted. b This study was conducted using lenacapavir 300 mg single dose administered orally. c Evaluated as a strong inducer of CYP3A, and an inducer of P-gp and UGT. d Fed. e Evaluated as a strong inhibitor of CYP3A, and an inhibitor UGT1A1 and P-gp. f Evaluated as a moderate inducer of CYP3A and an inducer of P-gp. g Evaluated as a strong inhibitor of CYP3A and an inhibitor of P-gp. h Evaluated as a strong inhibitor of CYP3A, and an inhibitor and inducer of P-gp. i This study was conducted using lenacapavir 600 mg single dose following a loading regimen of 600 mg twice daily for 2 days, single 600 mg doses of lenacapavir were administered with each co-administered medicinal product. j Evaluated as a P-gp substrate. k Tenofovir alafenamide is converted to tenofovir in vivo. l Evaluated as an OATP substrate. m Evaluated as an BCRP substrate. n Evaluated as a CYP3A substrate. o Major active metabolite of midazolam. p Evaluated as a strong inhibitor of CYP3A. q This study was conducted using voriconazole 400 mg loading dose twice daily for a day, followed by 200 mg maintenance dose twice daily.

פרטי מסגרת הכללה בסל
א. בשילוב עם תכשירים אנטירטרווירליים אחרים, לטיפול בנשאי HIV עמידים לטיפולים מרובים אשר לא השיגו דיכוי ויראלי בכל אפשרויות הטיפול הקיימות.ב. הטיפול לא יינתן בשילוב עם Ibalizumab. ג. מתן התרופה ייעשה לפי מרשם של מנהל מרפאה לטיפול באיידס, במוסד רפואי שהמנהל הכיר בו כמרכז AIDS.
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/02/2023
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
