Quest for the right Drug
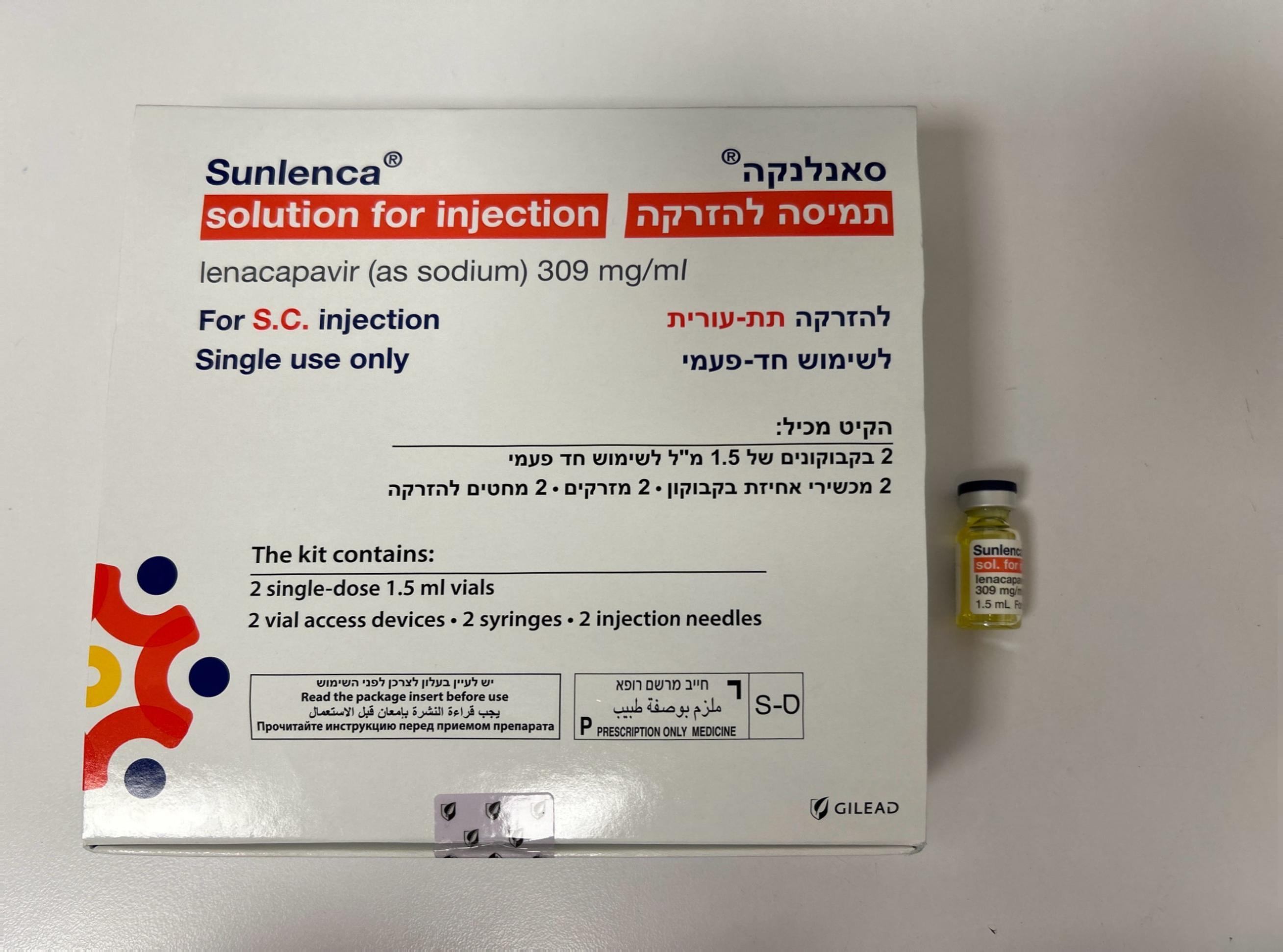
סאנלנקה תמיסה להזרקה SUNLENCA SOLUTION FOR INECTION (LENACAPAVIR AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Therapy should be prescribed by a physician experienced in the management of HIV infection. Each injection should be administered by a healthcare professional. Prior to starting lenacapavir, the healthcare professional should carefully select patients who agree to the required injection schedule and counsel patients about the importance of adherence to scheduled dosing visits to help maintain viral suppression and reduce the risk of viral rebound and potential development of resistance associated with missed doses. In addition, the healthcare professional should counsel patients about the importance of adherence to an optimised background regimen (OBR) to further reduce the risk of viral rebound and potential development of resistance. If Sunlenca is discontinued, it is essential to adopt an alternative, fully suppressive antiretroviral regimen where possible, no later than 28 weeks after the final injection of Sunlenca (see section 4.4). Posology Initiation On treatment Day 1 and Day 2, the recommended dose of Sunlenca is 600 mg per day taken orally. On treatment Day 8, the recommended dose is 300 mg taken orally. Then, on treatment Day 15, the recommended dose is 927 mg administered by subcutaneous injection. Oral tablets can be taken with or without food (see Sunlenca tablet Prescribing Information). Maintenance The recommended dose is 927 mg of Sunlenca administered by subcutaneous injection once every 6 months (26 weeks) from the date of the last injection (+/- 2 weeks). Table 1: Recommended treatment regimen for Sunlenca: initiation and maintenance dosing schedule Treatment time Dose of Sunlenca: initiation Day 1 600 mg orally (2 x 300 mg tablets) Day 2 600 mg orally (2 x 300 mg tablets) Day 8 300 mg orally (1 x 300 mg tablet) Day 15 927 mg subcutaneous injection (2 x 1.5 mL injectionsa) Dose of Sunlenca: maintenance Every 6 Months 927 mg subcutaneous injection (2 x 1.5 mL injectionsa) (26 weeks)b +/- 2 weeks a Two injections, each at a separate site in the abdomen. b From the date of the last injection. Missed dose During the maintenance period, if more than 28 weeks have elapsed since the last injection and if clinically appropriate to continue Sunlenca treatment, the regimen should be restarted from Day 1 (see table 1). Special populations Elderly No dose adjustment of Sunlenca is required in elderly patients (see section 5.2). Renal impairment No dose adjustment of Sunlenca is required in patients with mild, moderate, or severe renal impairment (creatinine clearance [CrCl] ≥ 15 mL/min). Sunlenca has not been studied in patients with end stage renal disease (CrCl < 15 mL/min or on renal replacement therapy) (see section 5.2), therefore Sunlenca should be used with caution in these patients. Hepatic impairment No dose adjustment of Sunlenca is required in patients with mild or moderate hepatic impairment (Child-Pugh Class A or B). Sunlenca has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) (see section 5.2), therefore Sunlenca should be used with caution in these patients. Paediatric population The safety and efficacy of Sunlenca in children under the age of 18 years old has not been established. No data are available. Method of administration For subcutaneous use. Sunlenca injections should be administered into the abdomen (two injections, each at a separate site) by a healthcare professional (see section 6.6). For instructions on preparation and administration, see ‘Instructions for Use’ in the package leaflet. ‘Instructions for Use’ are also available as a card in the injection kit.

פרטי מסגרת הכללה בסל
א. בשילוב עם תכשירים אנטירטרווירליים אחרים, לטיפול בנשאי HIV עמידים לטיפולים מרובים אשר לא השיגו דיכוי ויראלי בכל אפשרויות הטיפול הקיימות.ב. הטיפול לא יינתן בשילוב עם Ibalizumab. ג. מתן התרופה ייעשה לפי מרשם של מנהל מרפאה לטיפול באיידס, במוסד רפואי שהמנהל הכיר בו כמרכז AIDS.
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/02/2023
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
