Quest for the right Drug
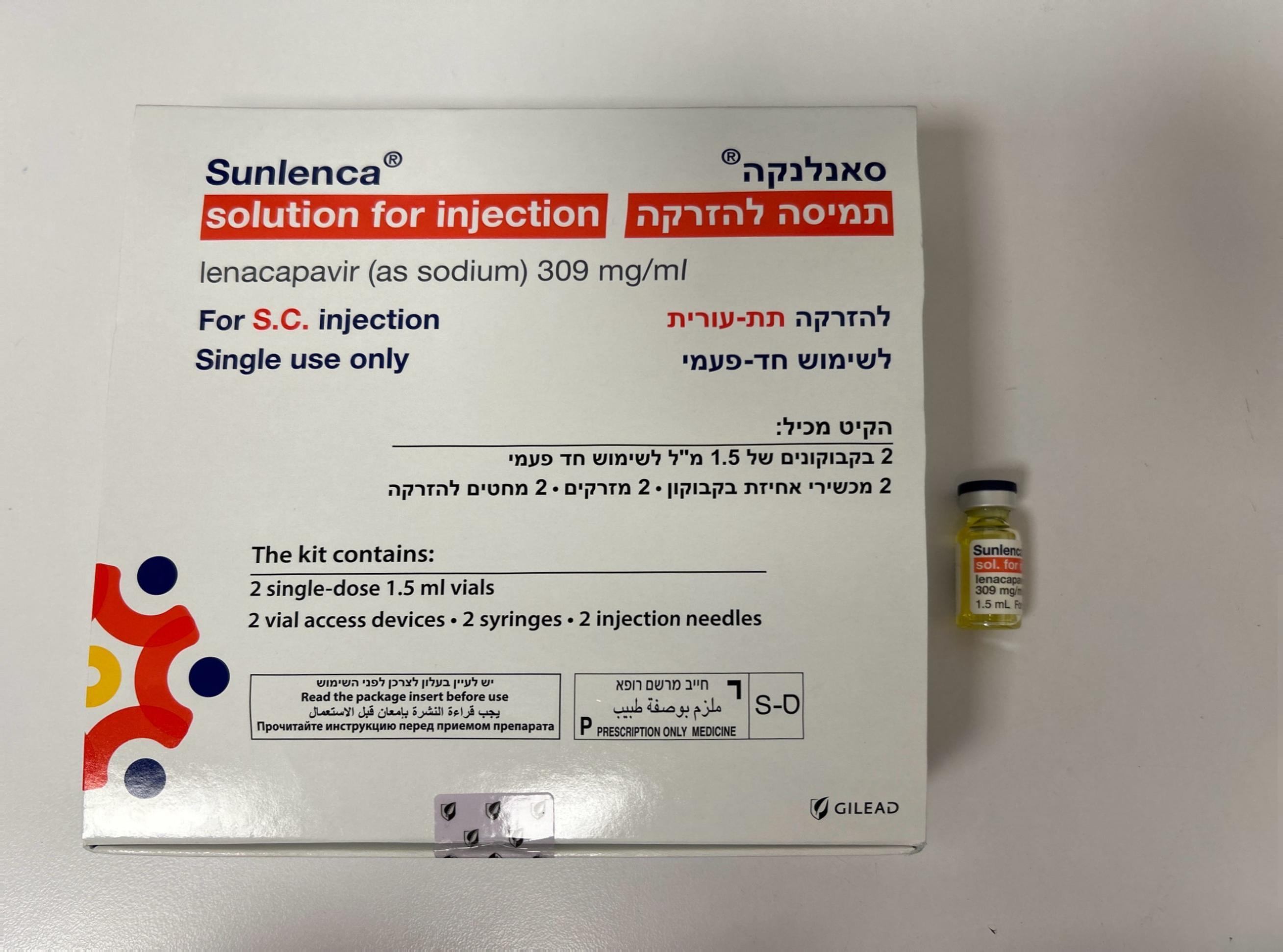
סאנלנקה תמיסה להזרקה SUNLENCA SOLUTION FOR INECTION (LENACAPAVIR AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Risk of resistance following treatment discontinuation If Sunlenca is discontinued, to minimise the risk of developing viral resistance it is essential to adopt an alternative, fully suppressive antiretroviral regimen where possible, no later than 28 weeks after the final injection of Sunlenca. If virologic failure is suspected, an alternative regimen should be adopted where possible. Use of other medicinal products after discontinuation of lenacapavir If Sunlenca is discontinued, residual concentrations of lenacapavir may remain in the systemic circulation of patients for prolonged periods. These concentrations may affect the exposures of other medicinal products (i.e. sensitive CYP3A substrates) that are initiated within 9 months after the last subcutaneous dose of Sunlenca (see section 4.5). These concentrations are not expected to affect the exposures of other antiretroviral agents that are initiated after discontinuation of Sunlenca. Immune Reconstitution Inflammatory Syndrome In HIV infected patients with severe immune deficiency at the time of institution of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first few weeks or months of initiation of CART. Relevant examples include cytomegalovirus retinitis, generalised and/or focal mycobacterial infections, and Pneumocystis jirovecii pneumonia. Any inflammatory symptoms should be evaluated and treatment instituted when necessary. Autoimmune disorders (such as Graves’ disease and autoimmune hepatitis) have also been reported to occur in the setting of immune reactivation; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment. Opportunistic infections Patients should be advised that Sunlenca or any other antiretroviral therapy does not cure HIV infection and that they may still develop opportunistic infections and other complications of HIV infection. Therefore, patients should remain under close clinical observation by physicians experienced in the treatment of patients with HIV associated diseases. Co-administration of other medicinal products Co-administration with medicinal products that are moderate inducers of CYP3A and P-gp (e.g. efavirenz) is not recommended (see section 4.5). Co-administration with medicinal products that are strong inhibitors of CYP3A, P-gp, and UGT1A1 together (i.e. all 3 pathways), such as atazanavir/cobicistat is not recommended (see section 4.5). Excipients This medicinal product contains less than 1 mmol sodium (23 mg) per injection, that is to say essentially ‘sodium-free’.
Effects on Driving
4.7 Effects on ability to drive and use machines Sunlenca is expected to have no or negligible influence on the ability to drive and use machines.

פרטי מסגרת הכללה בסל
א. בשילוב עם תכשירים אנטירטרווירליים אחרים, לטיפול בנשאי HIV עמידים לטיפולים מרובים אשר לא השיגו דיכוי ויראלי בכל אפשרויות הטיפול הקיימות.ב. הטיפול לא יינתן בשילוב עם Ibalizumab. ג. מתן התרופה ייעשה לפי מרשם של מנהל מרפאה לטיפול באיידס, במוסד רפואי שהמנהל הכיר בו כמרכז AIDS.
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/02/2023
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
