Quest for the right Drug
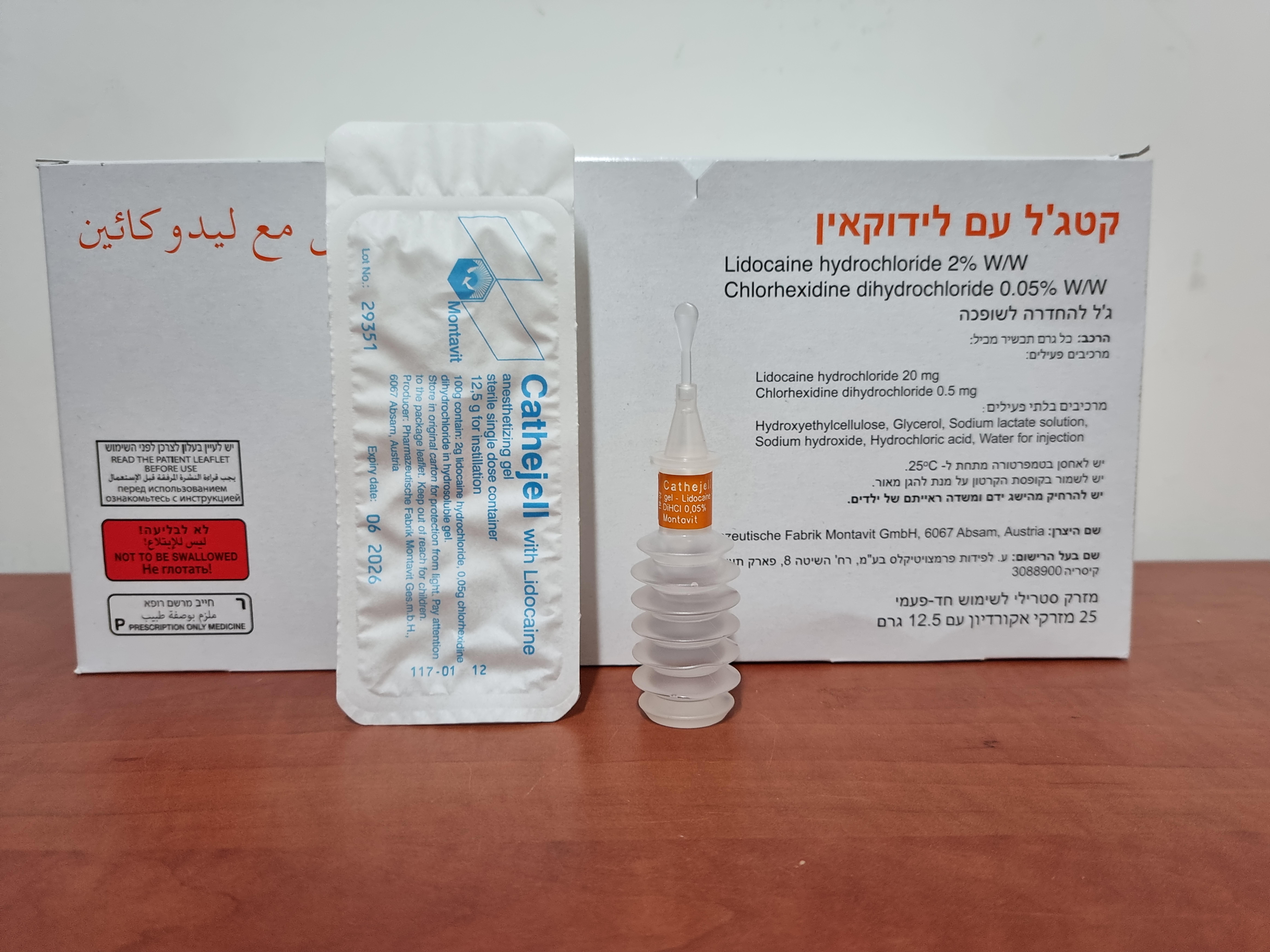
קטג'ל עם לידוקאין CATHEJELL WITH LIDOCAINE (CHLORHEXIDINE DIHYDROCHLORIDE, LIDOCAINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
לצינור השופכה : URETHRAL
צורת מינון:
ג'ל : GEL
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects An accurate estimation of the frequency of undesirable effects is not possible due to lacking data. The following incidence rates are used in the evaluation of undesirable effects: - Very common (≥1/10) - Common (≥1/100 to <1/10) - Uncommon (≥1/1,000 to <1/100) - Rare (≥ 1/10,000 to < 1/1,000) - Very rare (<1/10,000) - Not known (cannot be estimated from the available data) Adverse reactions rarely occur after the use of Cathejell with Lidocaine, provided that the product is used according to the dosage recommendations/recommendations for use and the necessary precautions are taken. In rare cases, local and/or systemic hypersensitivity reactions to lidocaine and/or chlorhexidine may occur. Systemic adverse reactions Systemic adverse reactions can be caused by high plasma levels, rapid absorption or overdose, as well as by hypersensitivity, idiosyncrasy or reduced tolerance, with possible onset of the following symptoms: CNS: nervousness, lightheadedness, blurred vision, tremor. These signs may or may not occur. In some patients, intoxication manifests itself in the form of sleepiness, unconsciousness or respiratory arrest. Cardiovascular: hypotension, bradycardia, asystole. For treatment of intoxication, see section 4.9. General disorders and administration site conditions Only low lidocaine blood levels are likely when used in urology; other systemic adverse reactions after instillation of Cathejell with Lidocaine into the urethra (see sections 4.4 and 4.9) are therefore normally not expected. Immune system disorders Allergic reactions to local amide-type anaesthetics (in extremely rare cases, anaphylactic shock) are rare. Bronchospasm, respiratory distress syndrome, cutaneous lesions, urticaria and oedema may occur as hypersensitivity to lidocaine or chlorhexidine and should be conventionally treated. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form (https://sideeffects.health.gov.il).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
