Quest for the right Drug
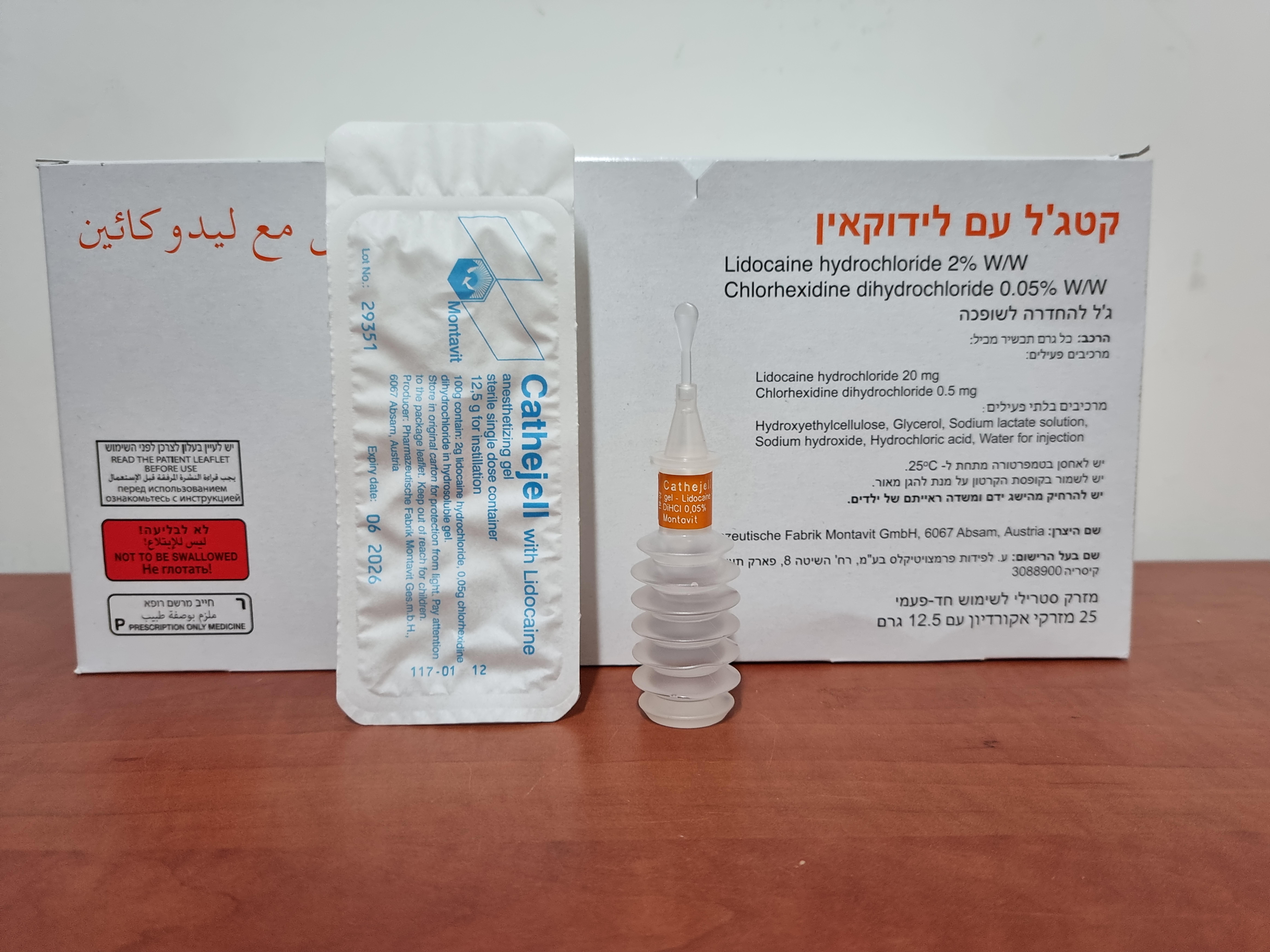
קטג'ל עם לידוקאין CATHEJELL WITH LIDOCAINE (CHLORHEXIDINE DIHYDROCHLORIDE, LIDOCAINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
לצינור השופכה : URETHRAL
צורת מינון:
ג'ל : GEL
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: local anaesthetics, amides, lidocaine, combinations ATC code: N01BB52 Mechansim of Action Cathejell with Lidocaine is a sterile and antiseptic gel with a local anaesthetic effect. Via local topical anaesthesia, Cathejell with Lidocaine produces anaesthesia of the mucous membranes, resulting in rapid symptomatic pain relief. The onset of action occurs as early as 5 to 10 minutes post administration and lasts for 20 to 30 minutes. In addition to pain relief, Cathejell with Lidocaine is thought to widely prevent urinary tract infections post catheterisation. Pharmacodynamic effects Lidocaine is a medically proven acid amide-type local anaesthetic. Lidocaine reversibly and locally inhibits conductivity of sensitive nerve fibres. Sensations are reduced sequentially in the order of cold/heat, touch and pressure. In topical anaesthesia, the onset of action generally occurs after about 3 to 5 min. The effect is reduced in inflamed tissue, due to the acidic pH value found there. In addition to use as an anaesthetic, lidocaine also has an antiarrhythmic effect. Unlike most other local anaesthetics, lidocaine has no vasodilatory effect. Chlorhexidine has antimicrobial activity against many gram-positive and gram- negative bacteria, as well as against a range of fungi and viruses. At the concentration in this product, it acts as a prophylactic against iatrogenic infections in the topical range of use.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Absorption Lidocaine is rapidly absorbed into the blood circulation via the mucous membranes. The amount absorbed following local mucosal application is dependent on the concentration and total administered dose, the specific site of application and duration of use. Local anaesthetics are generally absorbed most rapidly following intratracheal and bronchial use, which may lead to rapidly increasing or very high plasma concentrations with an increased risk of toxic symptoms. Lidocaine is rapidly absorbed from the gastrointestinal tract, although only little intact active substance enters the circulation due to metabolic degradation in the liver (“first-pass effect”). 45 to 60 minutes after intraurethral instillation of 10 to 40 ml lidocaine gel 2% (200 to 800 mg lidocaine), peak plasma concentrations of 0.06 to 0.2 µg/ml lidocaine are reached. These values are 7.5 to 27.5 times lower than the plasma concentrations of 1.5 to 5.5 µg/ml which are therapeutically relevant for the antiarrhythmic effect, and are lower than toxic plasma concentrations by a factor of 30 (5 to 8 µg/ml). It should be remembered that severe inflammation of the urethral mucosa and surface enlargement due to urethral dilation can lead to increased absorption of lidocaine. Chlorhexidine is absorbed in only very small amounts after topical use. Distribution Lidocaine has a volume of distribution of 1.3 to 1.6 L/kg; it is rapidly distributed to all tissues, especially highly vascularised organs, such as the lung, kidneys and skeletal muscles. Approximately 65% of lidocaine is bound to plasma proteins and alpha-1 acid glycoproteins (AAGs). As the number of AAGs increases with age, after trauma, surgery and in cases of cancer and chronic inflammation, yet decreases in renal and hepatic disease, protein binding in such cases may accordingly be increased or reduced. Lidocaine passes the blood-brain barrier. Following single intravenous administration, there is a biexponential decrease in the plasma concentration of lidocaine, with half-lives of approximately 8 minutes and of 1.6 hours. Biotransformation, elimination Lidocaine exhibits a marked first-pass metabolism. Approximately 90% of a lidocaine dose is rapidly dealkylated and metabolised in the liver to monoethylglycine xylidide (MEGX) or glycine xylidide (GX). As Na+ channel blockers, MEGX and GX are less active than lidocaine. Other metabolites are 2,6- xylidine and 4-hydroxy-2,6-xylidine. The terminal elimination half-life (t1/2) of 1.8 hours represents a primarily hepatic metabolism; it may be prolonged to up to 2.3 hours in the elderly. T1/2 of the active metabolites is 0.9 hours. The total plasma clearance of 0.95 L/min may be reduced in patients with heart failure or hepatic disease. Metabolite accumulation may occur in cases of renal insufficiency. Less than 5% of lidocaine is excreted unchanged with the urine. Kinetics in hepatic, renal and cardiac insufficiency Due to the rapid biotransformation of lidocaine in the liver, the half-life may be prolonged 2-fold or longer in patients with impaired hepatic function, e.g. to 4.5 to 6 hours in chronic cases of alcohol-induced liver damage. In patients with severe heart failure, the elimination half-life may be prolonged to 4 to 10 h. Renal insufficiency can lead to the accumulation of metabolites.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
