Quest for the right Drug
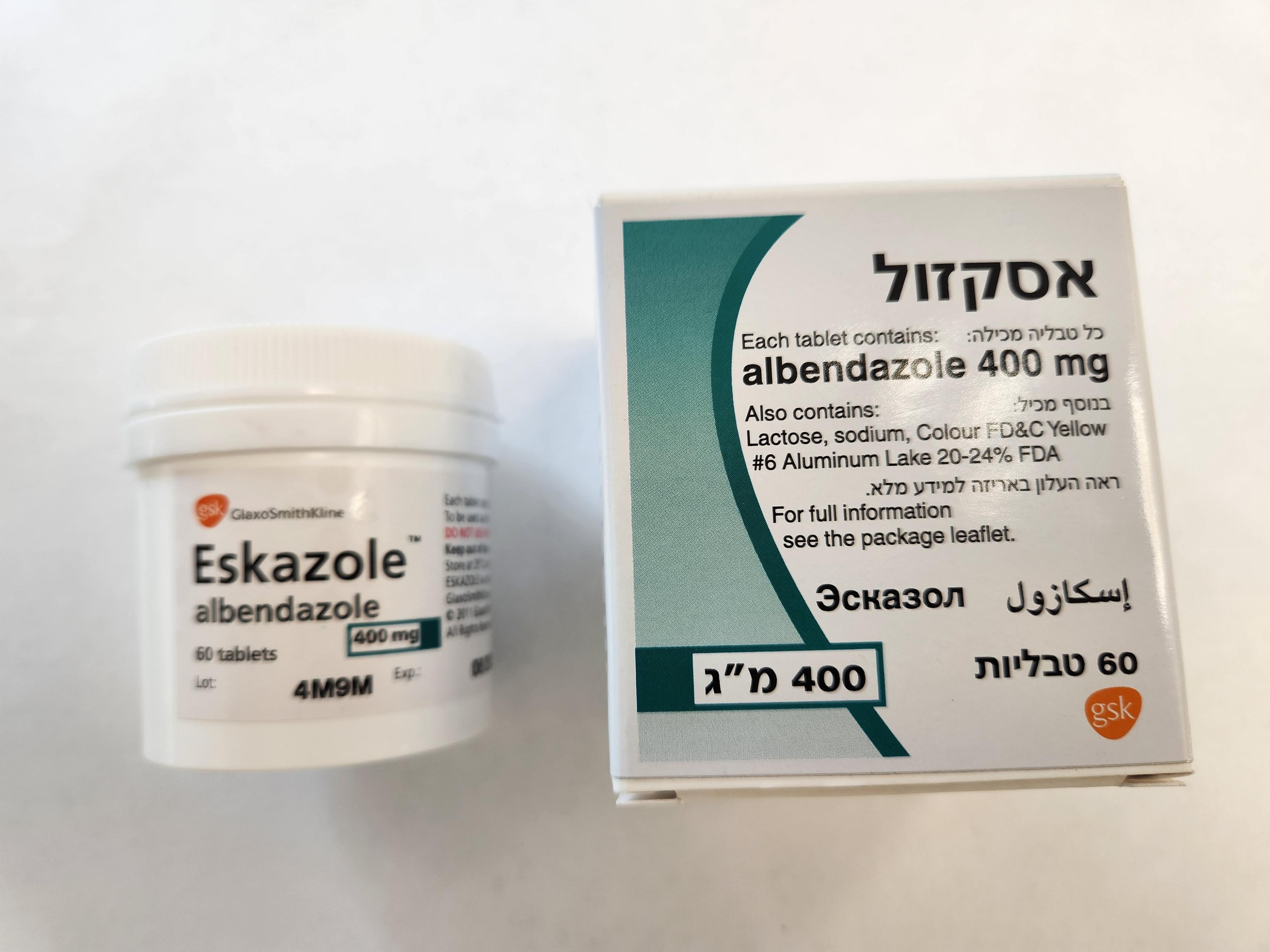
אסקזול ESKAZOLE (ALBENDAZOLE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8. Undesirable effects Data from large clinical studies were used to determine the frequency of adverse reactions classified as very common to rare. The frequencies assigned to all other reactions (i.e. those occurring in < 1/1000) were mainly determined using post-marketing data and refer to a reporting rate rather than a true frequency. The following convention (or agreement) has been used for the classification of frequency: Very common ≥1/10 Common ≥1/100 and <1/10 Uncommon ≥ 1/1000 and < 1/100 Rare ≥ 1/10,000 and < 1/1000 Very rare < 1/10,000 Use in systemic helminth infections: Blood and lymphatic system disorders Uncommon: leukopenia Very rare: pancytopenia, aplastic anemia, agranulocytosis Leukopenia has been associated with albendazole when treating patients with echinococcosis. Patients with hepatic disease, including hepatic echinococcosis, appear to be more susceptible to bone marrow suppression (see sections 4.2 and 4.8). Immune system disorders Uncommon: hypersensitivity reactions including rash, pruritus and urticaria Nervous system disorders Very common: headache Common: dizziness Gastrointestinal disorders Common: gastrointestinal disturbances (abdominal pain, nausea, vomiting) Gastrointestinal disturbances have been associated with albendazole when treating patients with echinococcosis. Hepatobiliary disorders Very common: mild to moderate elevation of hepatic enzymes. Uncommon: hepatitis Skin and subcutaneous tissue disorders Common: reversible alopecia (thinning of hair and moderate hair loss) Very rare: erythema multiforme, Stevens-Johnson syndrome General disorders and administration site conditions Common: fever Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il Additionally, you should also report to GSK Israel (il.safety@gsk.com).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לצרכן
03.05.18 - עלון לצרכן 27.02.20 - עלון לצרכן אנגלית 27.02.20 - עלון לצרכן עברית 27.02.20 - עלון לצרכן ערבית 03.01.23 - עלון לצרכן עברית 05.06.23 - עלון לצרכן אנגלית 05.06.23 - עלון לצרכן עברית 05.06.23 - עלון לצרכן ערבית 10.10.24 - עלון לצרכן עברית 30.11.11 - החמרה לעלון 19.04.17 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אסקזול