Quest for the right Drug
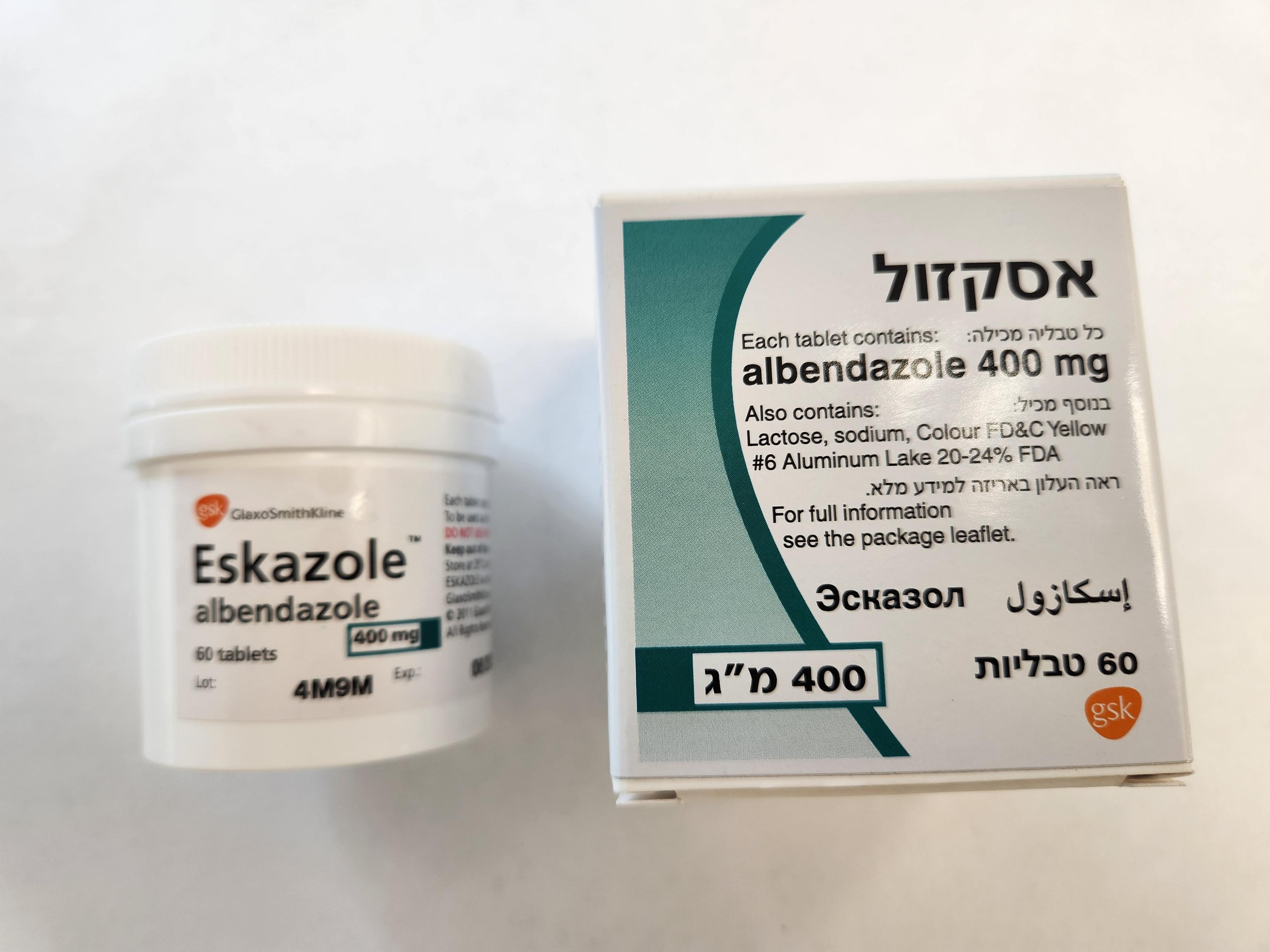
אסקזול ESKAZOLE (ALBENDAZOLE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1. Pharmacodynamic properties Pharmacotherapeutic group: benzimidazole derivatives, ATC code: P02CA03 Albendazole is a benzimidazole carbamate with antihelmintic and antiprotozoal effect against tissue and intestinal parasites. Albendazole exhibits larvicidal, ovicidal and vermicidal activity, and it is thought to exert its antihelmintic effect by inhibiting tubulin polymerization. This causes the disruption of the helminth metabolism, including energy depletion, which immobilizes and then kills the susceptible helminth. Albendazole is effective in the treatment of tissue parasites including cystic echinococcosis and alveolar echinococcosis caused by infestation of Echinococcus granulosus and Echinococcus multilocularis, respectively. Albendazole has been shown (in clinical trials) to eradicate cysts or significantly reduce cyst size in up to 80% of patients with cysts caused by Echinococcus granulosus. Where cysts have been investigated for viability following treatment with albendazole, 90% have been non- viable in laboratory or animal studies compared to only 10% of untreated cysts. Clinical experience with albendazole shows that during treatment of cysts due to Echinococcus multilocularis with albendazole, a minority of patients were considered cured and a majority had an improvement or stabilization of disease.
Pharmacokinetic Properties
5.2. Pharmacokinetic properties Absorption In man, albendazole is poorly absorbed (<5%) following oral administration. Albendazole rapidly undergoes first-pass metabolism in the liver, and is generally not detected in plasma. Albendazole sulfoxide is the primary metabolite, which is thought to be the active moiety in effectiveness against systemic tissue infections. The plasma half-life of albendazole sulfoxide is 8.5 hours. It has been reported that following oral administration of a single dose of 400 mg albendazole taken with breakfast, the pharmacologically active metabolite, albendazole sulfoxide, achieves plasma concentrations from 1.6 to 6.0 mol/L. The systemic pharmacological effect of albendazole is augmented if the dose is administered with a fatty meal, which enhances the absorption by approximately 5-fold. Excretion Albendazole and its metabolites appear to be principally excreted in bile, with only a small proportion appearing in urine. Excretion from cysts has been shown to occur after several weeks of prolonged treatment. Special patient populations • Elderly Although no studies have investigated the effect of age on albendazole sulfoxide pharmacokinetics, results in twenty-six patients with hydatid cyst (up to 79 years) suggest pharmacokinetics similar to those in young subjects. The number of elderly patients treated for either hydatid disease is limited, but no problems associated with an older population are observed. • Patients with renal impairment The pharmacokinetics of albendazole in patients with impaired renal function have not been studied. • Patients with hepatic impairment The pharmacokinetics of albendazole in patients with impaired hepatic function have not been studied.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לצרכן
03.05.18 - עלון לצרכן 27.02.20 - עלון לצרכן אנגלית 27.02.20 - עלון לצרכן עברית 27.02.20 - עלון לצרכן ערבית 03.01.23 - עלון לצרכן עברית 05.06.23 - עלון לצרכן אנגלית 05.06.23 - עלון לצרכן עברית 05.06.23 - עלון לצרכן ערבית 10.10.24 - עלון לצרכן עברית 30.11.11 - החמרה לעלון 19.04.17 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אסקזול