Quest for the right Drug
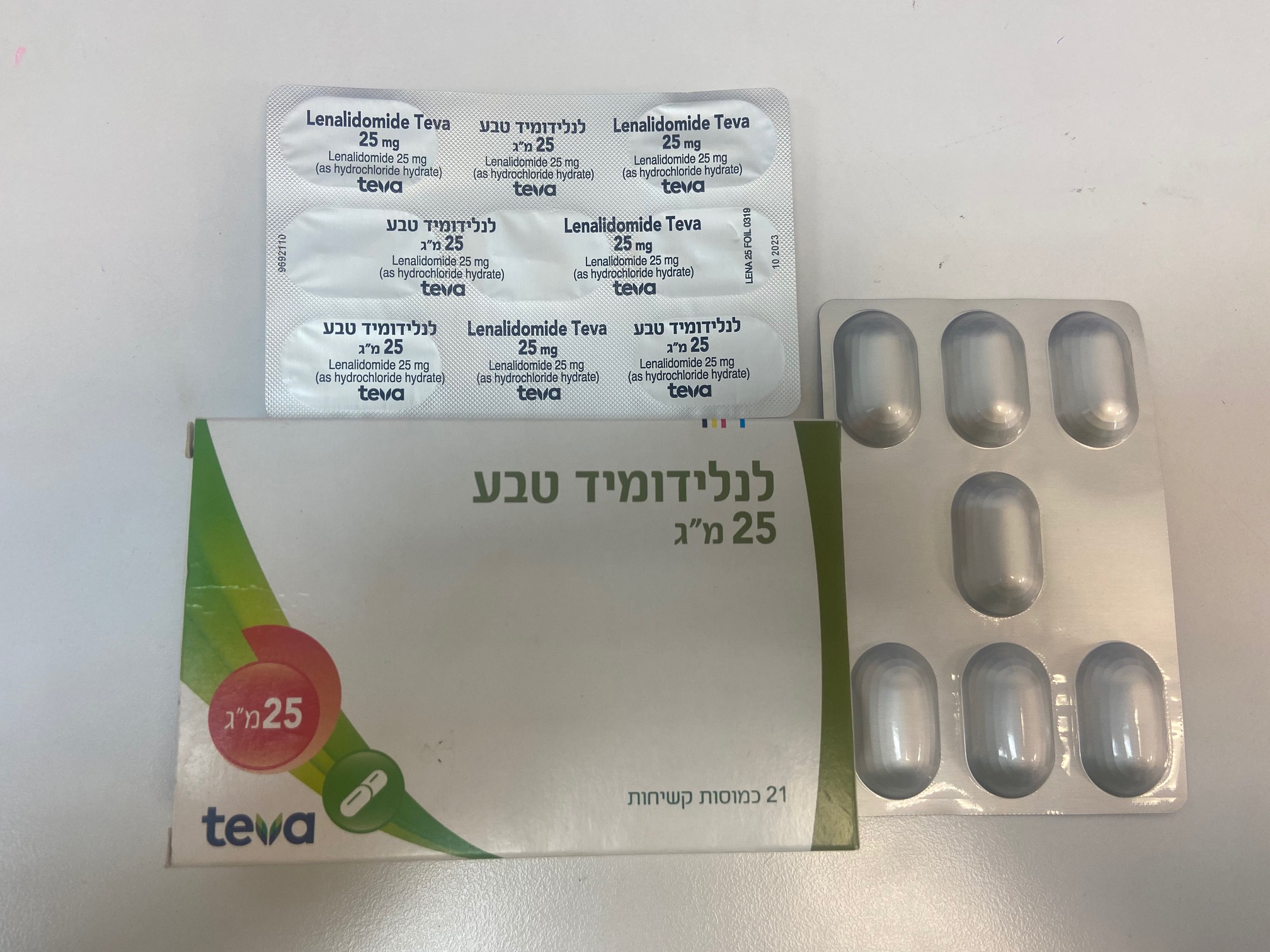
לנלידומיד טבע ® 25 מ"ג LENALIDOMIDE TEVA ® 25 MG (LENALIDOMIDE AS HYDROCHLORIDE HYDRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
קפסולה קשיחה : CAPSULE, HARD
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Lenalidomide Teva treatment should be supervised by a physician experienced in the use of anti-cancer therapies. For all indications described below: • Dose is modified based upon clinical and laboratory findings (see section 4.4). • Dose adjustments, during treatment and restart of treatment, are recommended to manage Grade 3 or 4 thrombocytopenia, neutropenia, or other Grade 3 or 4 toxicity judged to be related to Lenalidomide. • In case of neutropenia, the use of growth factors in patient management should be considered. • If less than 12 hours has elapsed since missing a dose, the patient can take the dose. If more than 12 hours has elapsed since missing a dose at the normal time, the patient should not take the dose, but take the next dose at the normal time on the following day. Posology Newly diagnosed multiple myeloma (NDMM) • Lenalidomide Teva in combination with dexamethasone until disease progression in patients who are not eligible for transplant Lenalidomide Teva treatment must not be started if the Absolute Neutrophil Count (ANC) is < 1.0 x 109/L, and/or platelet counts are < 50 x 109/L. Recommended dose The recommended starting dose of Lenalidomide Teva is 25 mg orally once daily on days 1 to 21 of repeated 28-day cycles. The recommended dose of dexamethasone is 40 mg orally once daily on days 1, 8, 15 and 22 of repeated 28-day cycles. Patients may continue Lenalidomide Teva and dexamethasone therapy until disease progression or intolerance. • Dose reduction steps Lenalidomide Tevaa Dexamethasonea Starting dose 25 mg 40 mg Dose level -1 20 mg 20 mg Dose level -2 15 mg 12 mg Dose level -3 10 mg 8 mg Dose level- 4 5 mg 4 mg Dose level -5 2.5 mg Not applicable ª Dose reduction for both products can be managed independently • Thrombocytopenia When platelets Recommended course Falls to < 25 x 109/L Stop Lenalidomide Teva dosing for remainder of cycleª Returns to ≥ 50 x 109/L Decrease by one dose level when dosing resumed at next cycle ª If Dose limiting toxicity (DLT) occurs on > day15 of a cycle, Lenalidomide Teva dosing will be interrupted for at least the remainder of the current 28-day cycle. • Absolute neutrophil count (ANC) – neutropenia When ANC Recommended courseª 9 First falls to < 0.5 x 10 /L Interrupt Lenalidomide Teva treatment 9 Returns to ≥ 1 x 10 /L when neutropenia is Resume Lenalidomide Teva at starting the only observed toxicity dose once daily When ANC Recommended courseª Returns to ≥ 0.5 x 109/L when dose- Resume Lenalidomide Teva at dose dependent haematological toxicities other level -1 once daily than neutropenia are observed For each subsequent drop below Interrupt Lenalidomide Teva treatment < 0.5 x 1109/L Returns to ≥ 0.5 x 109/L Resume Lenalidomide Teva at next lower dose level once daily. a At the physician’s discretion, if neutropenia is the only toxicity at any dose level, add granulocyte colony stimulating factor (G-CSF) and maintain the dose level of Lenalidomide Teva. For hematologic toxicity the dose of Lenalidomide Teva may be re-introduced to the next higher dose level (up to the starting dose) upon improvement in bone marrow function (no hematologic toxicity for at least 2 consecutive cycles: ANC ≥1,5 x 109/L with a platelet count ≥ 100 x 109/L at the beginning of a new cycle). • Lenalidomide Teva in combination with bortezomib and dexamethasone followed by Lenalidomide Teva and dexamethasone until disease progression in patients who are not eligible for transplant Initial treatment: Lenalidomide Teva in combination with bortezomib and dexamethasone Lenalidomide Teva in combination with bortezomib and dexamethasone must not be started if the ANC is < 1.0 x 109/L, and/or platelet counts are < 50 x 109/L. The recommended starting dose is Lenalidomide Teva 25 mg orally once daily days 1-14 of each 21-day cycle in combination with bortezomib and dexamethasone. Bortezomib should be administered via subcutaneous injection (1.3 mg/m2 body surface area) twice weekly on days 1, 4, 8 and 11 of each 21-day. Up to eight 21-day treatment cycles (24 weeks of initial treatment) are recommended. Lenalidomide Teva Bortezomib Dexamethasone 2 25 mg once daily on Days 1- 1.3 mg/m on Days 1, 4, 8 20 mg once daily orally on 14 of 21-day cycles and 11 of 21-day cycles Days 1, 2, 4, 5, 8, 9, 11 and 12 of 21-day cycles • Lenalidomide Teva in combination with bortezomib and dexamethasone followed by Lenalidomide Teva and dexamethasone until disease progression in patients who are eligible for transplant Up to eight 21-day or six 28-day treatment cycles (24 weeks of initial treatment) are recommended. Initial treatment: Lenalidomide in combination with bortezomib and dexamethasone Lenalidomide Teva in combination with bortezomib and dexamethasone must not be started if the ANC is < 1.0 x 109/L, and/or platelet counts are < 50 x 109/L. Lenalidomide Teva Bortezomib Dexamethasone 25 mg once daily on Days 1- 1.3 mg/m2 on Days 1, 4, 8 20 mg once daily on Days 1, 14 of 21-day cycles and 11 of 21-day cycles 2, 4, 5, 8, 9, 11 and 12 of 21-day cycles OR 25 mg once daily on Days 1- 1.3 mg/ m2 on Days 1, 4, 8 20 mg once daily on Days 1- 21 of 28-day cycles and 11 of 28-day cycles 4 and 9- 12 of 28-day cycles • Continued treatment: Autologous stem cell transplant For patients who proceed to autologous stem cell transplant, hematopoietic stem cell mobilization should occur within 4 cycles of initial therapy. Continued treatment: Lenalidomide Teva in combination with dexamethasone until progression Continue Lenalidomide Teva 25 mg orally once daily on days 1-21 of repeated 28-day cycles in combination with dexamethasone. Treatment should be continued until disease progression or unacceptable toxicity. • Dose reduction steps Lenalidomide Tevaa Starting dose 25 mg Dose level -1 20 mg Dose level -2 15 mg Dose level -3 10 mg Dose level- 4 5 mg Dose level -5 5 mg every other day ª Dose reduction for all products can be managed independently • Thrombocytopenia When platelets Recommended course Falls to < 30 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 50 x 109/L Resume Lenalidomide Teva at dose level -1 once daily For each subsequent drop Interrupt Lenalidomide Teva treatment below 30 x 109/L Returns to ≥ 50 x 109/L Resume Lenalidomide Teva at next lower dose level once daily • Absolute neutrophil count (ANC) – neutropenia When ANC Recommended coursea First falls to < 0.5 x 109/L or febrile neutropenia Interrupt Lenalidomide Teva treatment [< 1.0 x 109/L associated with fever (temperature ≥ 38.5°C)] Returns to ≥ 1 x 109/L when neutropenia is the Resume Lenalidomide Teva at starting only observed toxicity dose once daily When ANC Recommended coursea Returns to ≥ 0.5 x 109/L when dose-dependent Resume Lenalidomide Teva at dose level haematological toxicities other than neutropenia -1 once daily are observed For each subsequent drop Interrupt Lenalidomide Teva treatment below < 0.5 x 109/L Returns to ≥ 0.5 x 109/L Resume Lenalidomide Teva at next lower dose level once daily. a At the physician’s discretion, if neutropenia is the only toxicity at any dose level, add granulocyte colony stimulating factor (G-CSF) and maintain the dose level of Lenalidomide Teva. • Lenalidomide Teva in combination with melphalan and prednisone followed by Lenalidomide Teva maintenance in patients who are not eligible for transplant Lenalidomide Teva treatment must not be started if the ANC is < 1.5 x 109/L, and/or platelet counts are < 75 x 109/L. Recommended dose The recommended starting dose is Lenalidomide Teva 10 mg orally once daily on days 1 to 21 of repeated 28-day cycles for up to 9 cycles, melphalan 0.18 mg/kg orally on days 1 to 4 of repeated 28-day cycles, prednisone 2 mg/kg orally on days 1 to 4 of repeated 28-day cycles. Patients who complete 9 cycles or who are unable to complete the combination therapy due to intolerance are treated with Lenalidomide Teva monotherapy as follows: 10 mg orally once daily on days 1 to 21 of repeated 28-day cycles given until disease progression. • Dose reduction steps Lenalidomide Teva Melphalan Prednisone Starting dose 10 mgª 0.18 mg/kg 2 mg/kg Dose level -1 7.5 mg 0.14 mg/kg 1 mg/kg Dose level -2 5 mg 0.10 mg/kg 0.5 mg/kg Dose level -3 2.5 mg Not applicable 0.25 mg/kg ª If neutropenia is the only toxicity at any dose level, add granulocyte colony stimulating factor (G-CSF) and maintain the dose level of Lenalidomide Teva • Thrombocytopenia When platelets Recommended course First falls to < 25 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 25 x 109/L Resume Lenalidomide Teva and melphalan at dose level -1 For each subsequent drop Interrupt Lenalidomide Teva treatment below 30 x 109/L Returns to ≥ 30 x 109/L Resume Lenalidomide Teva at next lower dose level (dose level -2 or -3) once daily. • Absolute neutrophil count (ANC) - neutropenia When ANC Recommended courseª First falls to < 0.5 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 0.5 x 109/L when neutropenia is Resume Lenalidomide Teva at starting the only observed toxicity dose once daily Returns to ≥ 0.5 x 109/L when dose- dependent Resume Lenalidomide Teva at dose level haematological toxicities other than -1 once daily neutropenia are observed For each subsequent drop below Interrupt Lenalidomide Teva treatment < 0.5 x 109/L Returns to ≥ 0.5 x 109/L Resume Lenalidomide Teva at next lower dose level once daily. a At the physician’s discretion, if neutropenia is the only toxicity at any dose level, add granulocyte colony stimulating factor (G-CSF) and maintain the dose level of Lenalidomide Teva. • Lenalidomide Teva maintenance in patients who have undergone autologous stem cell transplantation (ASCT) Lenalidomide Teva maintenance should be initiated after adequate haematologic recovery following ASCT in patients without evidence of progression. Lenalidomide Teva must not be started if the ANC is < 1.0 x 109/L, and/or platelet counts are < 75 x 109/L. Recommended dose The recommended starting dose is Lenalidomide Teva 10 mg orally once daily continuously (on days 1 to 28 of repeated 28-day cycles) given until disease progression or intolerance. After 3 cycles of Lenalidomide Teva maintenance, the dose can be increased to 15 mg orally once daily if tolerated. • Dose reduction steps Starting dose (10 mg) If dose increased (15 mg)a Dose level -1 5 mg 10 mg Dose level -2 5 mg (days 1-21 every 28 days) 5 mg Dose level -3 Not applicable 5 mg (days 1-21 every 28 days) Do not dose below 5 mg (days 1-21 every 28 days) a After 3 cycles of Lenalidomide Teva maintenance, the dose can be increased to 15 mg orally once daily if tolerated. • Thrombocytopenia When platelets Recommended course Falls to < 30 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 30 x 109/L Resume Lenalidomide Teva at dose level -1 once daily For each subsequent drop Interrupt Lenalidomide Teva treatment below 30 x 109/L Returns to ≥ 30 x 109/L Resume Lenalidomide Teva at next lower dose level once daily • Absolute neutrophil count (ANC) - neutropenia When ANC Recommended coursea Falls to < 0.5 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 0.5 x 109/L Resume Lenalidomide Teva at dose level -1 once daily For each subsequent drop below < 0.5 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 0.5 x 109/L Resume Lenalidomide Teva at next lower dose level once daily a Atthe physician’s discretion, if neutropenia is the only toxicity at any dose level, add granulocyte colony stimulating factor (G-CSF) and maintain the dose level of Lenalidomide Teva. Multiple myeloma with at least one prior therapy Lenalidomide Teva treatment must not be started if the ANC < 1.0 x 109/L, and/or platelet counts < 75 x 109/L or, dependent on bone marrow infiltration by plasma cells, platelet counts < 30 x 109/L. Recommended dose The recommended starting dose of Lenalidomide Teva is 25 mg orally once daily on days 1 to 21 of repeated 28-day cycles. The recommended dose of dexamethasone is 40 mg orally once daily on days 1 to 4, 9 to 12, and 17 to 20 of each 28-day cycle for the first 4 cycles of therapy and then 40 mg once daily on days 1 to 4 every 28 days. Prescribing physicians should carefully evaluate which dose of dexamethasone to use, taking into account the condition and disease status of the patient. • Dose reduction steps Starting dose 25 mg Dose level -1 15 mg Dose level -2 10 mg Dose level -3 5 mg • Thrombocytopenia When platelets Recommended course First falls to < 30 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 30 x 109/L Resume Lenalidomide Teva at dose level -1 For each subsequent drop below 30 x 109/L Interrupt Lenalidomide Teva treatment Resume Lenalidomide Teva at next lower dose Returns to ≥ 30 x 109/L level (dose level -2 or -3) once daily. Do not dose below 5 mg once daily. • Absolute neutrophil count (ANC) – neutropenia When ANC Recommended coursea First falls to < 0.5 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 0.5 x 109/L when neutropenia is Resume Lenalidomide Teva at starting dose the only observed toxicity once daily Returns to ≥ 0.5 x 109/L when dose-dependent Resume Lenalidomide Teva at dose level -1 haematological toxicities other than once daily neutropenia are observed For each subsequent drop below < 0.5 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 0.5 x 109/L Resume Lenalidomide Teva at next lower dose level (dose level -1, -2 or -3) once daily. Do When ANC Recommended coursea not dose below 5 mg once daily. a At the physician’s discretion, if neutropenia is the only toxicity at any dose level, add granulocyte colony stimulating factor (G-CSF) and maintain the dose level of REVLI Lenalidomide Teva MID. Myelodysplastic syndromes (MDS) Lenalidomide Teva treatment must not be started if the ANC < 0.5 x 109/L and/or platelet counts < 25 x 109/L. Recommended dose The recommended starting dose of Lenalidomide Teva is 10 mg orally once daily on days 1 to 21 of repeated 28-day cycles. • Dose reduction steps Starting dose 10 mg once daily on days 1 to 21 every 28 days Dose level -1 5 mg once daily on days 1 to 28 every 28 days Dose level -2 2.5 mg once daily on days 1 to 28 every 28 days Dose level -3 2.5 mg every other day 1 to 28 every 28 days • Thrombocytopenia When platelets Recommended course Falls to < 25 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 25 x 109/L - < 50 x 109/L on at Resume Lenalidomide Teva at next lower dose least 2 occasions for ≥ 7 days or when the level (dose level -1, -2 or -3) platelet count recovers to ≥ 50 x 109/L at any time • Absolute neutrophil count (ANC) - neutropenia When ANC Recommended course Falls to < 0.5 x 109/L Interrupt Lenalidomide Teva treatment Returns to ≥ 0.5 x 109/L Resume Lenalidomide Teva at next lower dose level (dose level -1, -2 or -3) Discontinuation of Lenalidomide Teva Patients without at least a minor erythroid response within 4 months of therapy initiation, demonstrated by at least a 50% reduction in transfusion requirements or, if not transfused, a 1g/dl rise in haemoglobin, should discontinue Lenalidomide Teva treatment. Mantle cell lymphoma (MCL) Recommended dose The recommended starting dose of Lenalidomide Teva is 25 mg orally once daily on days 1 to 21 of repeated 28-day cycles. • Dose reduction steps Starting dose 25 mg once daily on days 1 to 21, every 28 days Dose Level -1 20 mg once daily on days 1 to 21, every 28 days Dose Level -2 15 mg once daily on days 1 to 21, every 28 days Dose Level -3 10 mg once daily on days 1 to 21, every 28 days Dose Level -4 5 mg once daily on days 1 to 21, every 28 days Dose Level -5 2.5 mg once daily on days 1 to 21, every 28 days1 5 mg every other day on days 1 to 21, every 28 days 1- In countries where the 2.5 mg capsule is available. • Thrombocytopenia When platelets Recommended course Falls to < 50 x 109/L Interrupt Lenalidomide Teva treatment and conduct Complete Blood Count (CBC) at least every 7 days Returns to ≥ 60 x 109/L Resume Lenalidomide Teva at next lower level (dose level -1) For each subsequent drop below 50 x 109/L Interrupt Lenalidomide Teva treatment and conduct the CBC at least every 7 days Returns to ≥60 x 109/L Resume Lenalidomide Teva at next lower level (dose level -2, -3, -4 or -5). Do not dose below dose level -5 • Absolute neutrophil count (ANC) - neutropenia When ANC Recommended course 9 Falls to < 1 x 10 /L for at least 7 days or Interrupt Lenalidomide Teva treatment and Falls to < 1 x 109/L with associated fever conduct the CBC at least every 7 days (body temperature ≥ 38.5°C) or Falls to < 0.5 x 109/L Returns to ≥ 1 x 109/L Resume Lenalidomide Teva at next lower dose level (dose level –1) 9 For each subsequent drop below 1 x 10 /L for Interrupt Lenalidomide Teva treatment at least 7 days or drop to < 1 x 109/L with associated fever (body temperature ≥ 38.5°C) or drop to < 0.5 x 109/L Resume Lenalidomide Teva at next lower dose level (dose level -2, -3, -4, -5). Do not dose 9 Returns to ≥1 x 10 /L below dose level -5 Follicular lymphoma (FL) Lenalidomide Teva treatment must not be started if the ANC is < 1 x 1109/L, and/or platelet count < 50 x 109/L, unless secondary to lymphoma infiltration of bone marrow. Recommended dose The recommended starting dose of Lenalidomide Teva is 20 mg, orally once daily on days 1 to 21 of repeated 28-day cycles for up to 12 cycles of treatment. The recommended starting dose of rituximab is 375 mg/m2 intravenously (IV) every week in Cycle 1 (days 1, 8, 15, and 22) and day 1 of every 28-day cycle for cycles 2 through 5. • Dose reduction steps Starting dose 20 mg once daily on days 1-21, every 28 days Dose Level -1 15 mg once daily on days 1-21, every 28 days Dose Level -2 10 mg once daily on days 1-21, every 28 days Dose Level -3 5 mg once daily on days 1-21, every 28 days For dose adjustments due to toxicity with rituximab, refer to the corresponding summary of product characteristics. • Thrombocytopenia When platelets Recommended course Falls to < 50 x 109/L Interrupt Lenalidomide Teva treatment and conduct CBC at least every 7 days Returns to ≥ 50 x 109/L Resume at next lower dose level (dose level -1) For each subsequent drop below 50 x 109/L Interrupt Lenalidomide Teva treatment and conduct CBC at least every 7 days Returns to ≥ 50 x 109/L Resume Lenalidomide Teva at next lower dose level (dose level -2, -3). Do not dose below dose level -3. • Absolute neutrophil count (ANC) - neutropenia When ANC Recommended course ª Falls < 1.0 x 109/L for at least 7 days or Interrupt Lenalidomide Teva treatment and Falls to < 1.0 x 109/L with associated fever conduct CBC at least every 7 days (body temperature ≥ 38.5°C) or Falls to < 0.5 x 109/L Returns to ≥ 1.0 x 109/L Resume Lenalidomide Teva at next lower dose level (dose level -1) 9 For each subsequent drop below 1.0 x 10 /L for Interrupt Lenalidomide Teva treatment and at least 7 days or drop to < 1.0 x 109/L with conduct CBC at least every 7 days associated fever (body temperature ≥ 38.5°C) or drop to < 0.5 x 109/L Resume Lenalidomide Teva at next lower dose 9 Returns to ≥1.0 x 10 /L level (dose level -2, -3). Do not dose below dose level-3 ª At the physician’s discretion, if neutropenia is the only toxicity at any dose level, add G-CSF Mantle cell lymphoma (MCL) or follicular lymphoma (FL) Tumour lysis syndrome (TLS) All patients should receive TLS prophylaxis (allopurinol, rasburicase or equivalent as per institutional guidelines) and be well hydrated (orally) during the first week of the first cycle or for a longer period if clinically indicated. To monitor for TLS, patients should have a chemistry panel drawn weekly during the first cycle and as clinically indicated. Lenalidomide Teva may be continued (maintain dose) in patients with laboratory TLS or Grade 1 clinical TLS, or at the physician’s discretion, reduce dose by one level and continue Lenalidomide Teva. Vigorous intravenous hydration should be provided and appropriate medical management according to the local standard of care, until correction of electrolyte abnormalities. Rasburicase therapy may be needed to reduce hyperuricaemia. Hospitalisation of the patient will be at physician’s discretion. In patients with Grade 2 to 4 clinical TLS, interrupt Lenalidomide Teva and obtain a chemistry panel weekly or as clinically indicated. Vigorous intravenous hydration should be provided and appropriate medical management according to the local standard of care, until correction of electrolyte abnormalities. Rasburicase therapy and hospitalisation will be at physician’s discretion. When the TLS resolves to Grade 0, restart Lenalidomide Teva at next lower dose per physician’s discretion (see section 4.4). Tumour flare reaction At the physician’s discretion, Lenalidomide Teva may be continued in patients with Grade 1 or 2 tumour flare reaction (TFR) without interruption or modification. At the physician’s discretion, therapy with non-steroidal anti- inflammatory drugs (NSAIDs), limited duration corticosteroids, and/or narcotic analgesics may be administered. In patients with Grade 3 or 4 TFR, withhold treatment with Lenalidomide Teva and initiate therapy with NSAIDs, corticosteroids and/or narcotic analgesics. When TFR resolves to ≤ Grade 1, restart Lenalidomide Teva treatment at the same dose level for the rest of the cycle. Patients may be treated for management of symptoms per the guidance for treatment of Grade 1 and 2 TFR (see section 4.4). All indications For other Grade 3 or 4 toxicities judged to be related to Lenalidomide Teva, treatment should be stopped and only restarted at next lower dose level when toxicity has resolved to ≤ Grade 2 depending on the physician’s discretion. Lenalidomide Teva interruption or discontinuation should be considered for Grade 2 or 3 skin rash. Lenalidomide Teva must be discontinued for angioedema, anaphylactic reaction, Grade 4 rash, exfoliative or bullous rash, or if Stevens- Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) or Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) is suspected, and should not be resumed following discontinuation from these reactions. Special populations • Paediatric population Lenalidomide Teva should not be used in children and adolescents from birth to less than 18 years because of safety concerns (see section 5.1). • Elderly Currently available pharmacokinetic data are described in section 5.2. Lenalidomide Teva has been used in clinical trials in multiple myeloma patients up to 91 years of age, in myelodysplastic syndromes patients up to 95 years of age and in mantle cell lymphoma patients up to 88 years of age (see section 5.1). Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it would be prudent to monitor renal function. Newly diagnosed multiple myeloma: patients who are not eligible for transplant Patients with newly diagnosed multiple myeloma aged 75 years and older should be carefully assessed before treatment is considered (see section 4.4). For patients older than 75 years of age treated with Lenalidomide Teva in combination with dexamethasone, the starting dose of dexamethasone is 20 mg once daily on days 1, 8, 15 and 22 of each 28-day treatment cycle. No dose adjustment is proposed for patients older than 75 years who are treated with Lenalidomide Teva in combination with melphalan and prednisone. In patients with newly diagnosed multiple myeloma aged 75 years and older who received Lenalidomide Teva, there was a higher incidence of serious adverse reactions and adverse reactions that led to treatment discontinuation. Lenalidomide Teva combined therapy was less tolerated in newly diagnosed multiple myeloma patients older than 75 years of age compared to the younger population. These patients discontinued at a higher rate due to intolerance (Grade 3 or 4 adverse events and serious adverse events), when compared to patients < 75 years. Multiple myeloma: patients with at least one prior therapy The percentage of multiple myeloma patients aged 65 or over was not significantly different between the Lenalidomide Teva /dexamethasone and placebo/dexamethasone groups. No overall difference in safety or efficacy was observed between these patients and younger patients, but greater pre-disposition of older individuals cannot be ruled out. Myelodysplastic syndromes For myelodysplastic syndromes patients treated with Lenalidomide Teva, no overall difference in safety and efficacy was observed between patients aged over 65 and younger patients. Mantle cell lymphoma For mantle cell lymphoma patients treated with Lenalidomide Teva, no overall difference in safety and efficacy was observed between patients aged 65 years or over compared with patients aged under 65 years of age. Follicular lymphoma For follicular lymphoma patients treated with Lenalidomide Teva in combination with rituximab, the overall rate of adverse events is similar for patients aged 65 years or over compared with patients under 65 years of age. No overall difference in efficacy was observed between the two age groups. • Patients with renal impairment Lenalidomide Teva is primarily excreted by the kidney; patients with greater degrees of renal impairment can have impaired treatment tolerance (see section 4.4). Care should be taken in dose selection and monitoring of renal function is advised. No dose adjustments are required for patients with mild renal impairment and multiple myeloma, myelodysplastic syndromes, mantle cell lymphoma, or follicular lymphoma. The following dose adjustments are recommended at the start of therapy and throughout treatment for patients with moderate or severe impaired renal function or end stage renal disease. There are no phase 3 trial experiences with End Stage Renal Disease (ESRD) (CLcr < 30 mL/min, requiring dialysis). Multiple myeloma Renal function (CLcr) Dose adjustment Moderate renal impairment 10 mg once daily1 (30 CLcr < 50 mL/min) Severe renal impairment 7.5 mg once daily2 (CLcr < 30 mL/min, not requiring dialysis) 15 mg every other day End Stage Renal Disease (ESRD) 5 mg once daily. On dialysis days, the dose (CLcr < 30 mL/min, requiring dialysis) should be administered following dialysis. 1. The dose may be escalated to 15 mg once daily after 2 cycles if patient is not responding to treatment and is tolerating the treatment. 2. In countries where the 7.5 mg capsule is available. Myelodysplastic syndromes Renal function (CLcr) Dose adjustment Moderate renal impairment (30 5 mg once daily Starting dose CLcr < 50 mL/min) (days 1 to 21 of repeated 28-day cycles) 2.5 mg once daily Dose level -1* (days 1 to 28 of repeated 28-day cycles) 2.5 mg once every other day Dose level -2* (days 1 to 28 of repeated 28-day cycles) Severe renal impairment 2.5 mg once daily (CLcr < 30 mL/min, not Starting dose (days 1 to 21 of repeated 28-day cycles) requiring dialysis) 2.5 mg every other day Dose level -1* (days 1 to 28 of repeated 28-day cycles) 2.5 mg twice a week Dose level -2* (days 1 to 28 of repeated 28-day cycles) End Stage Renal Disease 2.5 mg once daily (ESRD) (CLcr < 30 mL/min, Starting dose (days 1 to 21 of repeated 28-day cycles) requiring dialysis) 2.5 mg every other day Dose level -1* (days 1 to 28 of repeated 28-day cycles) On dialysis days, the dose 2.5 mg twice a week should be administered Dose level -2* (days 1 to 28 of repeated 28-day cycles) following dialysis. * Recommended dose reduction steps during treatment and restart of treatment to manage Grade 3 or 4 neutropenia or thrombocytopenia, or other Grade 3 or 4 toxicity judged to be related to Lenalidomide Teva , as described above. Mantle cell lymphoma Renal function (CLcr) Dose adjustment (days 1 to 21 of repeated 28-day cycles) Moderate renal impairment 10 mg once daily1 (30 CLcr < 50 mL/min) Severe renal impairment 7.5 mg once daily2 (CLcr < 30 mL/min, not requiring dialysis) 15 mg every other day End Stage Renal Disease (ESRD) 5 mg once daily. On dialysis days, the dose (CLcr < 30 mL/min, requiring dialysis) should be administered following dialysis. 1. The dose may be escalated to 15 mg once daily after 2 cycles if patient is not responding to treatment and is tolerating the treatment. 2. In countries where the 7.5 mg capsule is available. Follicular lymphoma Renal function (CLcr) Dose adjustment (days 1 to 21 of repeated 28-day cycles) Moderate renal impairment 10 mg once daily1, 2 (30 CLcr < 60 mL/min) Severe renal impairment No data available3 (CLcr < 30 mL/min, not requiring dialysis) End Stage Renal Disease (ESRD) (CLcr < 30 No data available3 mL/min, requiring dialysis) 1. The dose may be escalated to 15 mg once daily after 2 cycles if the patient has tolerated therapy. 2. For patients on a starting dose of 10 mg, in case of dose reduction to manage Grade 3 or 4 neutropenia or thrombocytopenia, or other Grade 3 or 4. Toxicity judged to be related to Lenalidomide Teva do not dose below 5 mg every other day or 2.5 mg once daily. 3. Patients with severe renal impairment or ESRD were excluded from study. After initiation of Lenalidomide Teva therapy, subsequent Lenalidomide Teva dose modification in renally impaired patients should be based on individual patient treatment tolerance, as described above. • Patients with hepatic impairment Lenalidomide Teva has not formally been studied in patients with impaired hepatic function and there are no specific dose recommendations. Method of administration Oral use. Lenalidomide Teva capsules should be taken orally at about the same time on the scheduled days. The capsules should not be opened, broken or chewed. The capsules should be swallowed whole, preferably with water, either with or without food. It is recommended to press only on one end of the capsule to remove it from the blister thereby reducing the risk of capsule deformation or breakage.

פרטי מסגרת הכללה בסל
א. התרופה האמורה תינתן לטיפול במקרים האלה: 1. מיאלומה נפוצה ובהתקיים אחד מאלה: א. חולה שטרם קיבל טיפול למחלתו ואינו מועמד להשתלת מח עצם.הטיפול יינתן בשילוב עם Dexamethasone או בשילוב עם Dexamethasone ו-Bortezomib.ב. מונותרפיה כטיפול אחזקה במאובחן חדש לאחר השתלת מח עצם.ג. חולה שמחלתו עמידה או נשנית לאחר מיצוי קו טיפול אחד שכלל אחד מהשניים - BORTEZOMIB או THALIDOMIDE, אלא אם כן לחולה הייתה הורית נגד לאחד מהטיפולים האמורים. על אף האמור בפסקה זו הטיפול בתכשיר ייפסק בחולה העונה על אחד מאלה: א. בחולה שמחלתו התקדמה לאחר שני מחזורי טיפול מלאים או ארבעה מחזורי טיפול חלקיים. ב. חולה שפיתח תופעות לוואי קשות לטיפול. הטיפול בתכשיר יינתן לחולה שטרם טופל ב-Lenalidomide למחלה זו. 2. תסמונת מיאלודיספלסטית ברמת חומרה low או intermediate-1 עם הפרעה ציטוגנטית מסוג deletion 5q. 3. בשילוב עם Rituximab, לטיפול בלימפומה פוליקולרית כקו טיפול מתקדם.ב. מתן התרופה האמורה ייעשה לפי מרשם של מומחה בהמטולוגיה.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| בשילוב עם Rituximab, לטיפול בלימפומה פוליקולרית כקו טיפול מתקדם | 01/03/2021 | המטולוגיה | לימפומה פוליקולרית, Follicular lymphoma | |
| מיאלומה נפוצה ובהתקיים אחד מאלה: א. חולה שטרם קיבל טיפול למחלתו ואינו מועמד להשתלת מח עצם. הטיפול יינתן בשילוב עם Dexamethasone או בשילוב עם Dexamethasone ו-Bortezomib. ב. כטיפול אחזקה במאובחן חדש לאחר השתלת מח עצם. | 16/01/2019 | המטולוגיה | מיאלומה נפוצה, Multiple myeloma | |
| תסמונת מיאלודיספלסטית ברמת חומרה low או intermediate-1 עם הפרעה ציטוגנטית מסוג deletion 5q. | 10/01/2012 | המטולוגיה | MDS, Myelodysplastic syndrome | |
| א. התרופה האמורה תינתן לטיפול במיאלומה נפוצה בחולה שמחלתו עמידה או נשנית לאחר מיצוי קו טיפול אחד שכלל אחד מהשניים – Bortezomib או Thalidomide, אלא אם לחולה הייתה הורית נגד לאחד מהטיפולים האמורים. ב. על אף האמור בפסקת משנה א הטיפול בתכשיר ייפסק: 1. בחולה שמחלתו התקדה לאחר שני מחזורי טיפול מלאים או ארבעה מחזורי טיפול חלקיים. 2. חולה שפיתח תופעות לוואי קשות לטיפול. ג. הטיפול בתכשיר יינתן לחולה שטרם טופל ב-Lenalidomide למחלה זו. | 23/01/2011 | המטולוגיה | מיאלומה נפוצה, Multiple myeloma | |
| א. התרופה האמורה תינתן לטיפול במיאלומה נפוצה בחולה שמחלתו עמידה או נשנית לאחר לפחות שני קווי טיפול שכללו BORTEZOMIB ו-THALIDOMIDE, אלא אם לחולה הייתה הורית נגד לאחד מהטיפולים האמורים. ב. על אף האמור בפסקת משנה (א) הטיפול בתכשיר ייפסק: 1. בחולה שמחלתו התקדמה לאחר שני מחזורי טיפול מלאים או ארבעה מחזורי טיפול חלקיים. 2. חולה שפיתח תופעות לוואי קשות לטיפול. ג. הטיפול בתכשיר יינתן לחולה שטרם טופל ב-LENALIDOMIDE למחלה זו. | 03/01/2010 | המטולוגיה | מיאלומה נפוצה, Multiple myeloma |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
03/01/2010
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לרופא
26.09.24 - עלון לרופאעלון מידע לצרכן
11.05.21 - עלון לצרכן אנגלית 11.05.21 - עלון לצרכן עברית 11.05.21 - עלון לצרכן ערבית 11.05.21 - עלון לצרכן 11.05.21 - עלון לצרכן אנגלית 11.05.21 - עלון לצרכן 11.05.21 - עלון לצרכן אנגלית 11.05.21 - עלון לצרכן עברית 01.11.21 - עלון לצרכן אנגלית 01.11.21 - עלון לצרכן עברית 01.11.21 - עלון לצרכן עברית 01.11.21 - עלון לצרכן ערבית 01.11.21 - עלון לצרכן 13.08.19 - עלון לצרכן אנגלית 13.01.21 - עלון לצרכן אנגלית 24.05.22 - עלון לצרכן אנגלית 13.08.19 - עלון לצרכן עברית 24.05.22 - עלון לצרכן עברית 13.08.19 - עלון לצרכן ערבית 13.01.21 - עלון לצרכן ערבית 24.05.22 - עלון לצרכן ערבית 23.08.23 - עלון לצרכן אנגלית 24.09.24 - עלון לצרכן עברית 12.10.20 - החמרה לעלון 19.12.21 - החמרה לעלון 24.09.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
לנלידומיד טבע ® 25 מ"ג