Quest for the right Drug
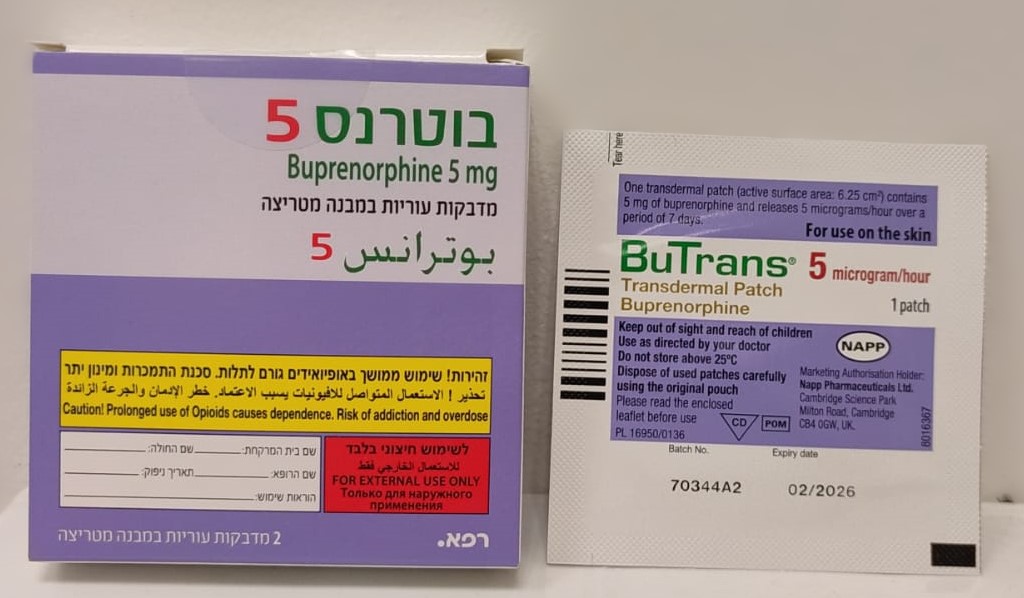
בוטרנס 5 BUTRANS 5 (BUPRENORPHINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
בין-עורי : TRANSDERMAL
צורת מינון:
מדבקות במבנה מטריצה : PATCHES MATRIX
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2. Posology and method of administration Posology BuTrans should be administered once per week on the same day each week. BuTrans is not suitable for the treatment of acute pain. Patients aged 18 years and over: The lowest BuTrans dose (BuTrans 5 μg/h transdermal patch) should be used as the initial dose. Consideration should be given to the previous opioid history of the patient (see section 4.5) as well as to the current general condition and medical status of the patient. Prior to starting treatment with opioids, a discussion should be held with patients to put in place a strategy for ending treatment with buprenorphine in order to minimise the risk of addiction and drug withdrawal syndrome (see section 4.4). Titration: During initiation of treatment with BuTrans, short-acting supplemental analgesics may be required as needed until analgesic efficacy with BuTrans is attained. The dose of BuTrans may be titrated upwards as indicated after 3 days, when the maximum effect of a given dose is established. Subsequent dosage increases may then be titrated based on the need for supplemental pain relief and the patient’s analgesic response to the patch. To increase the dose, a larger patch should replace the patch that is currently being worn, or a combination of patches should be applied in different places to achieve the desired dose. It is recommended that no more than two patches are applied at the same time, up to (and including) a maximum total dose of 40 microgram/hour. A new patch should not be applied to the same skin site for the subsequent 3-4 weeks (see section 5.2). Patients should be carefully and regularly monitored to assess the optimum dose and duration of treatment. Conversion from opioids: BuTrans can be used as an alternative to treatment with other opioids. Such patients should be started on the lowest available dose (BuTrans 5 μg/h transdermal patch) and continue taking short-acting supplemental analgesics during titration, as required. Paediatric population: The safety and efficacy of BuTrans in children below 18 years of age has not been established. No data are available. Elderly: No dosage adjustment of BuTrans is required in elderly patients. Renal impairment: No special dose adjustment of BuTrans is necessary in patients with renal impairment. Hepatic impairment: Buprenorphine is metabolised in the liver. The intensity and duration of its action may be affected in patients with impaired liver function. Therefore patients with hepatic insufficiency should be carefully monitored during treatment with BuTrans. Patients with severe hepatic impairment may accumulate buprenorphine during BuTrans treatment. Consideration of alternate therapy should be considered, and BuTrans should be used with caution, if at all, in such patients. Method of administration Route of administration: Transdermal patch to be worn for 7 days. The patch must not be divided or cut into pieces. Patch application: In order to ensure effective analgesia of buprenorphine and to minimise the potential for skin reactions (see section 4.4.) the following directions of use should be followed. BuTrans should be applied to non-irritated, intact skin of the upper outer arm, upper chest, upper back or the side of the chest, but not to any parts of the skin with large scars. BuTrans should be applied to a relatively hairless or nearly hairless skin site. If none are available, the hair at the site should be cut with scissors, not shaven. If the application site must be cleaned, it should be done with clean water only. Soaps, alcohol, oils, lotions or abrasive devices must not be used. The skin must be dry before the patch is applied. BuTrans should be applied immediately after removal from the sealed sachet. Following removal of the protective layer, the transdermal patch should be pressed firmly in place with the palm of the hand for approximately 30 seconds, making sure the contact is complete, especially around the edges. If the edges of the patch begin to peel off, the edges may be taped down with suitable skin tape to ensure a 7 day period of wear. The patch should be worn continuously for 7 days. Bathing, showering, or swimming should not affect the patch. If a patch falls off, a new one should be applied and worn for 7 days. Duration of administration: BuTrans should under no circumstances be administered for longer than absolutely necessary. If long-term pain treatment with BuTrans is necessary in view of the nature and severity of the illness, then careful and regular monitoring should be carried out (if necessary with breaks in treatment) to establish whether and to what extent further treatment is necessary. Discontinuation: After removal of the patch, buprenorphine serum concentrations decrease gradually and thus the analgesic effect is maintained for a certain amount of time. This should be considered when therapy with BuTrans is to be followed by other opioids. As a general rule, a subsequent opioid should not be administered within 24 hours after removal of the patch. At present, only limited information is available on the starting dose of other opioids administered after discontinuation of the transdermal patch (see section 4.5). Patients with fever or exposed to external heat: While wearing the patch, patients should be advised to avoid exposing the application site to external heat sources, such as heating pads, electric blankets, hot water bottles, heat lamps, sauna, hot tubs, and heated water beds, etc., as an increase in absorption of buprenorphine may occur. When treating febrile patients, one should be aware that fever may also increase absorption resulting in increased plasma concentrations of buprenorphine and thereby increased risk of opioid reactions.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/2000
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
