Quest for the right Drug
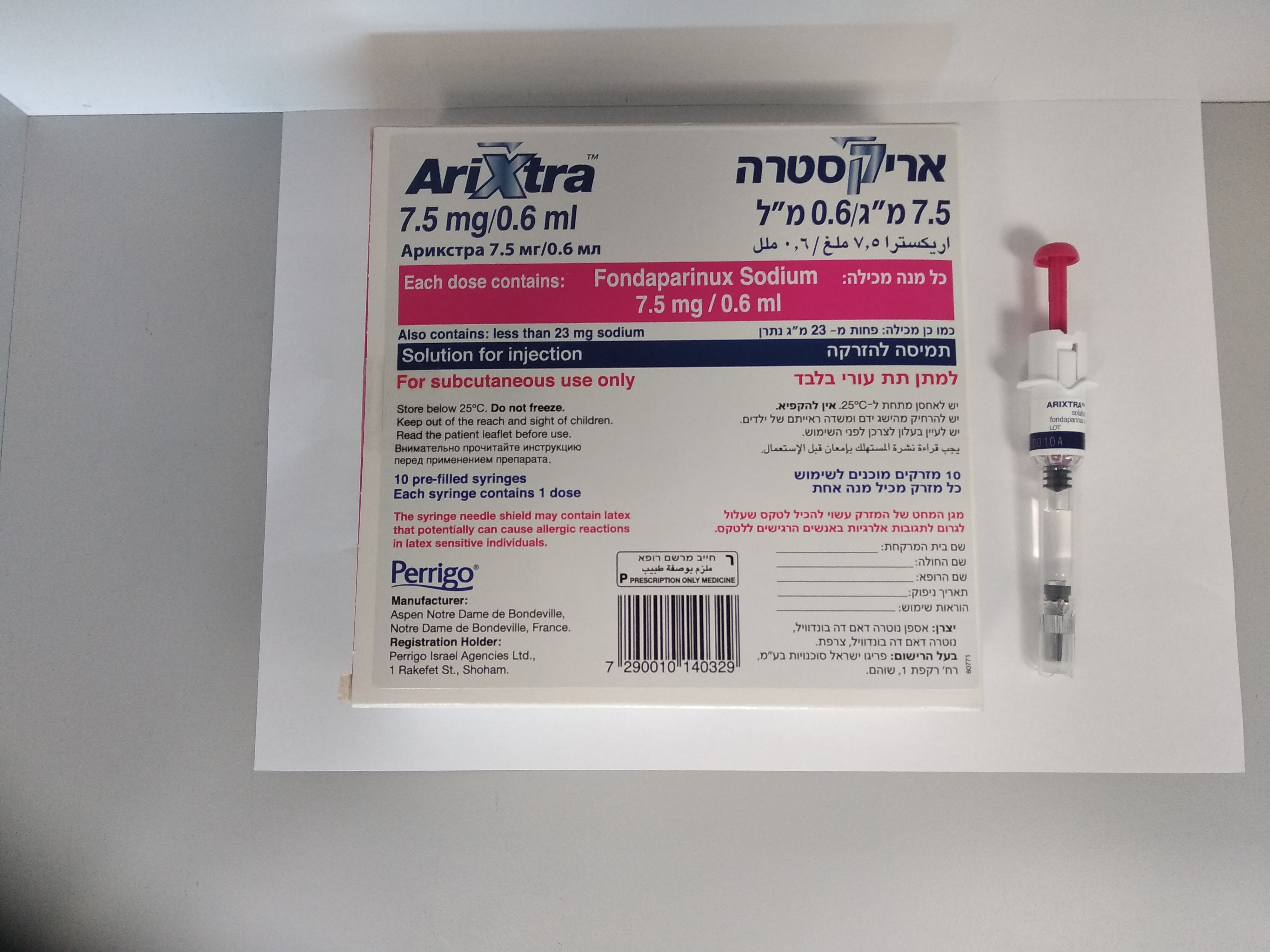
אריקסטרה 7.5 מ"ג / 0.6 מ"ל ARIXTRA 7.5 MG/0.6 ML (FONDAPARINUX SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The most commonly reported serious adverse reactions reported with fondaparinux are bleeding complications (various sites including rare cases of intracranial/ intracerebral and retroperitoneal bleedings). Fondaparinux should be used with caution in patients who have an increased risk of haemorrhage (see section 4.4). The safety of fondaparinux has been evaluated in: - 3,595 patients undergoing major orthopaedic surgery of the lower limbs treated up to 9 days (Arixtra 2.5 mg/0.5 ml) - 327 patients undergoing hip fracture surgery treated for 3 weeks following an initial prophylaxis of 1 week (Arixtra 2.5 mg/0.5 ml) - 1,407 patients undergoing abdominal surgery treated up to 9 days (Arixtra 2.5 mg/0.5 ml) - 425 medical patients who are at risk for thromboembolic complications treated up to 14 days (Arixtra 2.5 mg/0.5 ml) - 10,057 patients undergoing treatment of UA or NSTEMI ACS (Arixtra 2.5 mg/0.5 ml) - 6,036 patients undergoing treatment of STEMI ACS (Arixtra 2.5 mg/0.5 ml) - 2,517 patients treated for Venous Thrombo-Embolism and treated with fondaparinux for an average of 7 days (Arixtra 7.5 mg/0.6 ml). These adverse reactions should be interpreted within the surgical or medical context of the indications. The adverse event profile reported in the ACS program is consistent with the adverse drug reactions identified for VTE prophylaxis. Adverse reactions are listed below by system organ class and frequency. Frequencies are defined as: very common (≥ 1/10), common (≥1/100, < 1/10), uncommon (≥ 1/1,000, < 1/100), rare (≥ 1/10,000, <1/1,000), very rare (<1/10,000). System organ class common uncommon rare MedDRA (≥ 1/100, <1/10) (≥ 1/1,000, <1/100) (≥ 1/10,000, <1/1,000) Infections and post-operative wound infestations infections anaemia, post-operative thrombocytopenia, retroperitoneal Blood and lymphatic haemorrhage, thrombocythaemia, bleeding*, system disorders uterovaginal platelet abnormal, hepatic, intracranial/ haemorrhage*, coagulation disorder intracerebral bleeding* haemoptysis, haematuria, haematoma, gingival bleeding, purpura, epistaxis, gastrointestinal bleeding, hemarthrosis*, ocular bleeding*, bruise* allergic reaction Immune system (including very rare disorders reports of angioedema, anaphylactoid/ anaphylactic reaction) hypokalaemia, non- Metabolism and protein- nutrition disorders nitrogen (Npn) increased1* headache anxiety, confusion, Nervous system dizziness, somnolence, disorders vertigo hypotension Vascular disorders Respiratory, thoracic dyspnoea coughing and mediastinal disorders Gastrointestinal nausea, vomiting abdominal pain, disorders dyspepsia, gastritis, constipation, diarrhoea Hepatobiliary disorders abnormal liver function bilirubinaemia tests, hepatic enzymes increased Skin and subcutaneous rash erythematous, tissue disorders pruritus oedema, oedema reaction at injection General disorders and peripheral, pain, fever, site, administration site chest pain, wound leg pain, fatigue, conditions secretion flushing, syncope, hot flushes, oedema genital (1) Npn stands for non-protein-nitrogen such as urea, uric acid, amino acid, etc. * ADRs occurred at higher dose 7.5 mg/0.6 ml. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il Additionally, you can also report to Padagis via the following address: Padagis.co.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לצרכן
09.07.19 - עלון לצרכן אנגלית 17.04.22 - עלון לצרכן עברית 09.07.19 - עלון לצרכן ערבית 03.01.23 - עלון לצרכן אנגלית 03.01.23 - עלון לצרכן עברית 03.01.23 - עלון לצרכן ערבית 07.11.24 - עלון לצרכן עברית 15.01.12 - החמרה לעלון 11.08.13 - החמרה לעלון 01.11.18 - החמרה לעלון 07.11.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אריקסטרה 7.5 מ"ג / 0.6 מ"ל