Quest for the right Drug
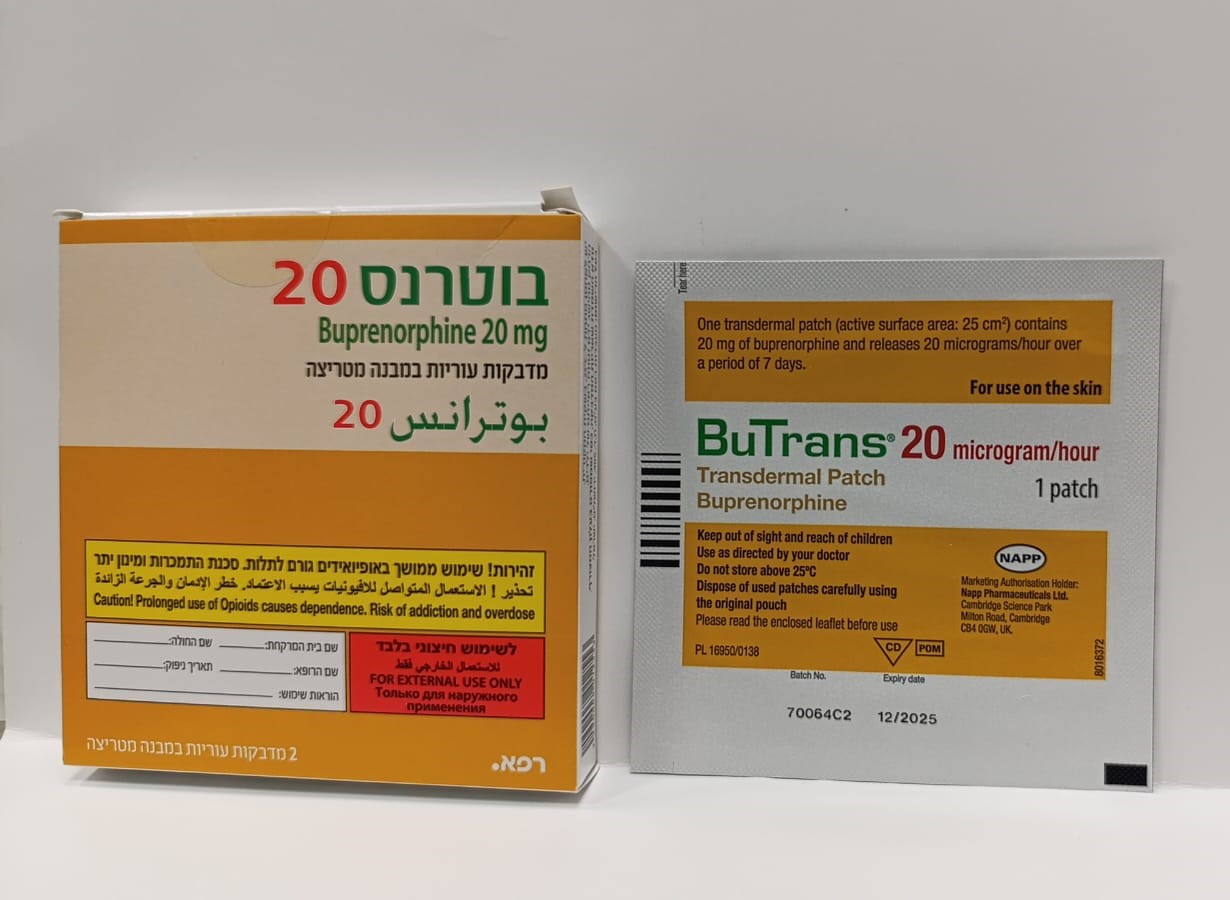
בוטרנס 20 BUTRANS 20 (BUPRENORPHINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
בין-עורי : TRANSDERMAL
צורת מינון:
מדבקות במבנה מטריצה : PATCHES MATRIX
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Interactions : אינטראקציות
4.5. Interaction with other medicinal products and other forms of interaction BuTrans must not be used concomitantly with MAOIs or in patients who have received MAOIs within the previous two weeks (see section 4.3). BuTrans should be used cautiously when co-administered with serotonergic medicinal products, such as selective serotonin re-uptake inhibitors (SSRIs), serotonin norepinephrine re-uptake inhibitors (SNRIs) or tricyclic antidepressants as the risk of serotonin syndrome, a potentially life-threatening condition, is increased (see section 4.4). Effect of other active substances on the pharmacokinetics of buprenorphine: Buprenorphine is primarily metabolised by glucuronidation and to a lesser extent (about 30%) by CYP3A4. Concomitant treatment with CYP3A4 inhibitors may lead to elevated plasma concentrations with intensified efficacy of buprenorphine. Studies with the CYP3A4 inhibitor ketoconazole did not produce clinically relevant increases in mean maximum (Cmax) or total (AUC) buprenorphine exposure following BuTrans with ketoconazole as compared to BuTrans alone. The interaction between buprenorphine and CYP3A4 enzyme inducers has not been studied. Co-administration of BuTrans and enzyme inducers (e.g., phenobarbital, carbamazepine, phenytoin and rifampicin) could lead to increased clearance which might result in reduced efficacy. Reductions in hepatic blood flow induced by some general anaesthetics (e.g., halothane) and other medicinal products may result in a decreased rate of hepatic elimination of buprenorphine. Pharmacodynamic interactions: BuTrans should be used cautiously with: Other central nervous system depressants: other opioid derivatives (analgesics and antitussives containing e.g., morphine, dextropropoxyphene, codeine, dextromethorphan or noscapine). Certain antidepressants, sedative H1- receptor antagonists, alcohol, anxiolytics, neuroleptics, clonidine and related substances. These combinations increase the CNS depressant activity. Benzodiazepines: This combination can potentiate respiratory depression of central origin (see section 4.4 and BOX Warning). At typical analgesic doses buprenorphine is a partial mu-receptor agonist but it is described to function as a pure mu receptor agonist. In BuTrans clinical studies subjects receiving full mu agonist opioids (up to 90 mg oral morphine or oral morphine equivalents per day) were transferred to BuTrans. There were no reports of abstinence syndrome or opioid withdrawal during conversion from entry opioid to BuTrans (see section 4.4).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/2000
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
