Quest for the right Drug
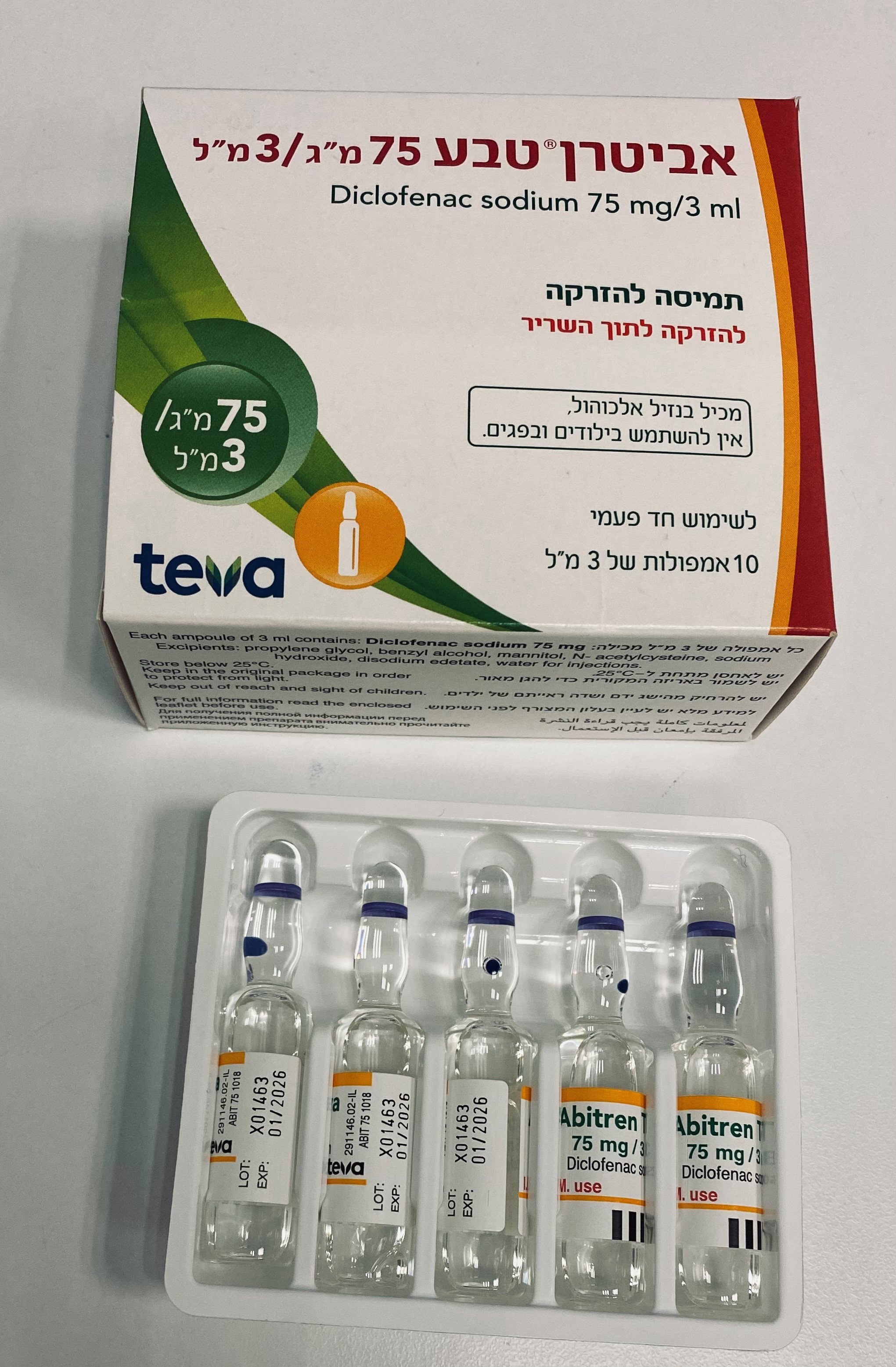
אביטרן טבע ® 75מ"ג/3 מ"ל ABITREN TEVA ® 75 MG/3 ML (DICLOFENAC SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pregnancy & Lactation : הריון/הנקה
4.6 Fertility, pregnancy and lactation Pregnancy Inhibition of prostaglandin synthesis may adversely affect the pregnancy and/or the embryo/foetal development. Data from epidemiological studies suggest an increased risk of miscarriage and or cardiac malformation and gastroschisis after use of a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk for cardiovascular malformation was increased from less than 1% up to approximately 1.5%. The risk is believed to increase with dose and duration of therapy. In animals, administration of a prostaglandin synthesis inhibitor has shown to result in increased pre-and post-implantation loss and embryo-foetal lethality. In addition, increased incidences of various malformations, including cardiovascular, have been reported in animals given a prostaglandin synthesis inhibitor during organogenetic period. From the 20th week of pregnancy onward, Abitren use may cause oligohydramnios resulting from foetal renal dysfunction. This may occur shortly after treatment initiation and is usually reversible upon discontinuation. In addition, there have been reports of ductus arterious constriction following treatment in the second trimester, most of which resolved after treatment cessation. Therefore, during the first and second trimester of pregnancy, Abitren should not be given unless clearly necessary. If Abitren Teva 75mg/3 ml is used by a woman attempting to conceive, or during the 1st or 2nd trimester of pregnancy, the dose should be kept as low as possible and duration of treatment as short as possible. Rarely, use of NSAIDs, including Abitren, after 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes were seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some postmarketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required. Use of NSAIDs after 20 weeks gestation should be limited. If the benefits outweight the risks to the fetus and the treatment is necessary after 20 weeks gestation, limit Abitren use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if Abitren full dose treatment extends beyond 5 days. Discontinue Abitren if oligohydramnios occurs and follow up according to clinical practice. Antenatal monitoring for oligohydramnios and ductus arteriosus constriction should be considered after exposure to diclofenac for several days from gestational week 20 onward. Voltarol should be discontinued if oligohydramnios or ductus arteriosus constriction is found. During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the foetus to: • Cardiopulmonary toxicity (with premature constriction/closure of the ductus arteriosus and pulmonary hypertension). • Renal dysfunction (see above). The mother and the neonate, at the end of the pregnancy, to: • Possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses. • Inhibition of uterine contractions resulting in delayed or prolonged labour. Consequently, Abitren Teva 75mg/3 ml is contraindicated during the third trimester of pregnancy. Lactation Like other NSAIDs, diclofenac passes into breast milk in small amounts. Therefore, diclofenac should not be administered during breast-feeding in order to avoid undesirable effects in the infant (see section 5.2 Pharmacokinetic properties). Female fertility As with other NSAIDs, the use of diclofenac may impair female fertility and is not recommended in women attempting to conceive. In women who may have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of diclofenac should be considered. See also section 4.4 Special warnings and precautions for use, regarding female fertility.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
