Quest for the right Drug
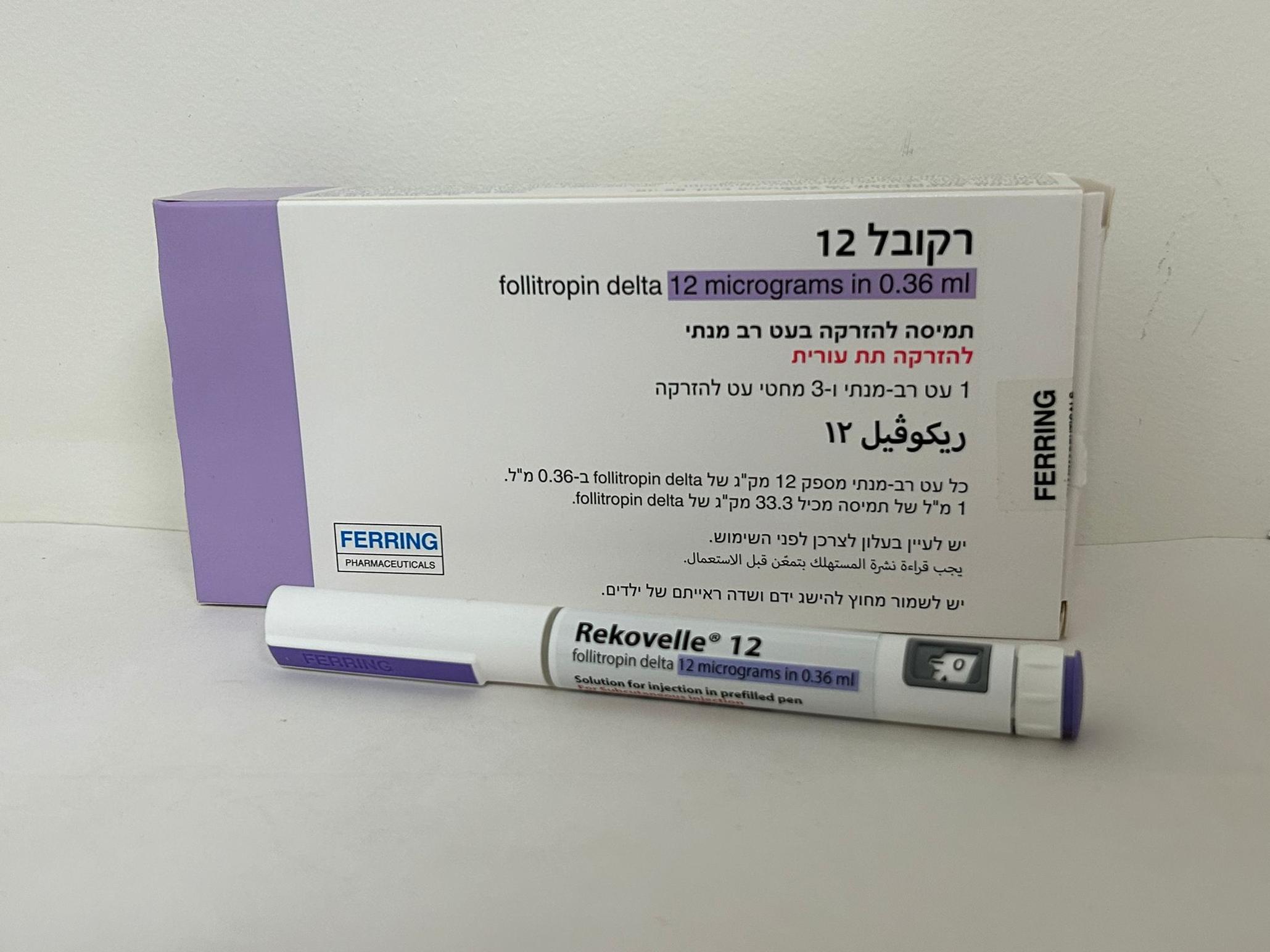
רקובל REKOVELLE (FOLLITROPIN DELTA)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of safety profile The most frequently reported adverse reactions during treatment with REKOVELLE are OHSS headache, , , pelvic pain, nausea, and fatigue. The frequency of these adverse reactions might decrease with repeated treatment cycles, as this has been observed in clinical trials. Tabulated list of adverse reactions The table below (Table 2) displays the adverse reactions experienced in clinical trials by patients treated with REKOVELLE) using the algorithm-based dosing regimen. Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. Table 2 Adverse reactions in pivotal clinical trials Common Uncommon System Organ Class (≥1/100 to <1/10) (≥1/1,000 to <1/100) Psychiatric disorders Mood swings Nervous system disorders Headache Somnolence Dizziness Gastrointestinal disorders Nausea Diarrhoea Vomiting Constipation Abdominal discomforta Reproductive system and breast OHSS Vaginal haemorrhage disorders Pelvic painb Breast discomfortc General disorders and Fatigue administration site conditions a Abdominal discomfort includes abdominal pain/distention. b Pelvic pain includes pelvic discomfort and adnexa uteri pain. c Breast discomfort includes breast pain, breast swelling, breast tenderness and/or nipple pain. Description of selected adverse reactions OHSS is an intrinsic risk of the ovarian stimulation. Known gastrointestinal symptoms associated with OHSS include abdominal pain, discomfort, and distension, nausea, vomiting and diarrhoea. Ovarian torsion and thromboembolic events are known to be rare complications of ovarian stimulation treatment (see section 4.4). Immunogenicity in terms of development of anti-FSH antibodies is a potential risk of gonadotropin therapy (see section 5.1). Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
