Quest for the right Drug
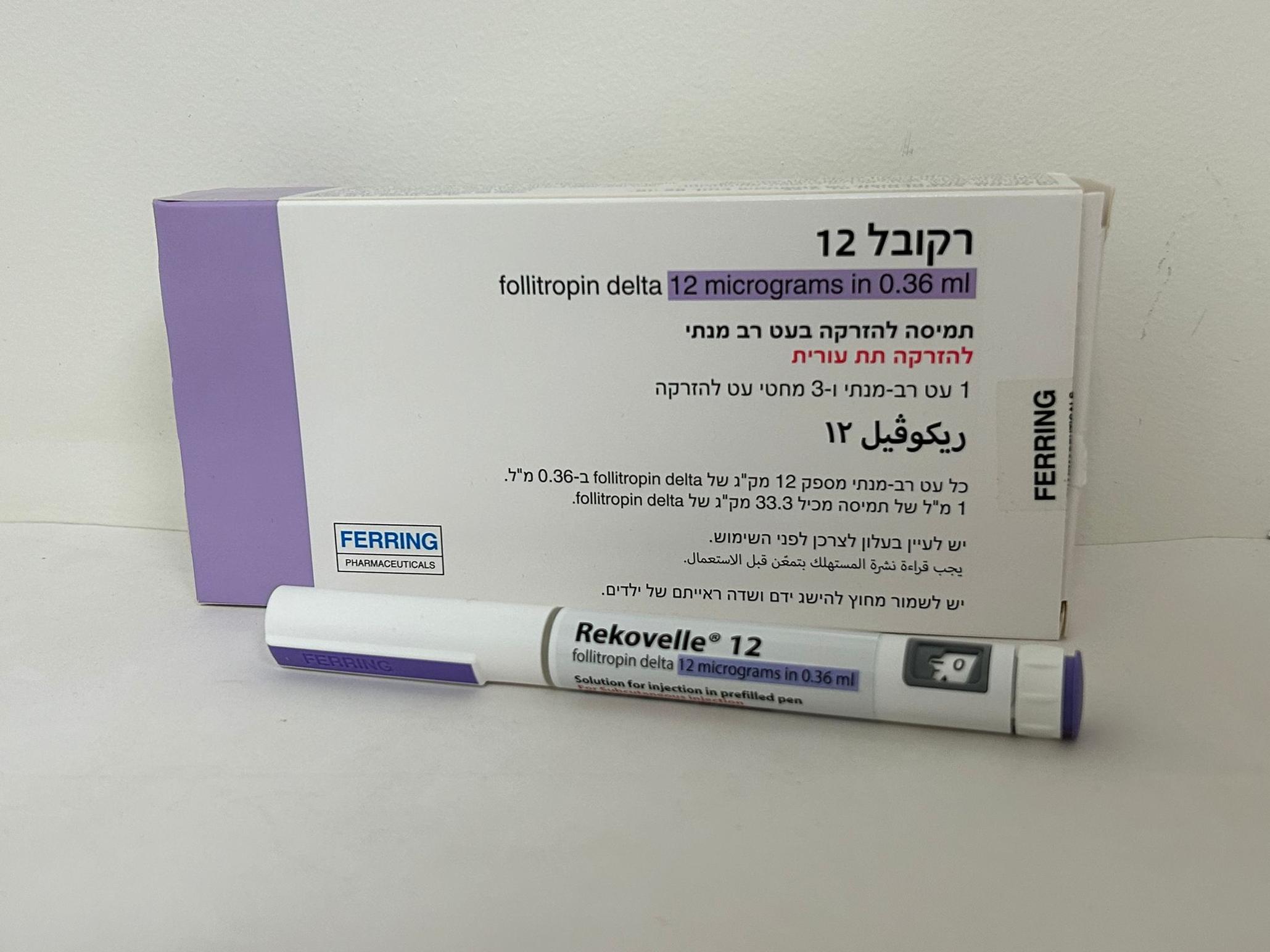
רקובל REKOVELLE (FOLLITROPIN DELTA)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Sex hormones and modulators of the genital systems, gonadotropins, ATC code: G03GA10 Mechanism of action The most important effect resulting from parenteral administration of FSH is the development of multiple mature follicles. Follitropin delta is a recombinant human FSH. The amino acid sequences of the two FSH subunits in follitropin delta are identical to the endogenous human FSH sequences. Because follitropin delta is produced in the human cell line PER.C6, the glycosylation profile is different from follitropin alfa and follitropin beta. Pharmacodynamic effects Following daily administration of equal IU doses of REKOVELLE and follitropin alfa as determined in the rat in vivo bioassay (Steelman-Pohley assay), higher ovarian response (i.e. estradiol, inhibin B and follicular volume) was observed in patients after administration of REKOVELLE compared to follitropin alfa. As the rat bioassay might not fully reflect the potency of the FSH in REKOVELLE in humans, REKOVELLE is dosed in micrograms and not in IU. The clinical trial data suggest that a daily dose of 10.0 [95% CI 9.2; 10.8] micrograms REKOVELLE provides, for the majority of patients, an ovarian response close to that obtained with 150 IU/day follitropin alfa. The number of oocytes retrieved increases with the dose of REKOVELLE and serum AMH concentration. Conversely, increasing body weight leads to a decrease in the number of oocytes retrieved (only clinically relevant for REKOVELLE doses below 12 micrograms). The resulting REKOVELLE dosing regimen is in section 4.2. Clinical efficacy and safety The ESTHER-1 trial was a randomised, assessor-blinded, controlled trial in 1,326 IVF/ICSI patients. The trial compared the individualised dosing regimen of REKOVELLE where the daily dose is established for each patient and fixed throughout stimulation with no adjustments (see section 4.2) to follitropin alfa filled-by-mass at a starting dose of 11 micrograms (150 IU) for the first five days followed by dose adjustments from day 6 of stimulation based on follicular development in a GnRH antagonist protocol. The patients were up to 40 years of age and had regular menstrual cycles presumed to be ovulatory. Single blastocyst transfer on day 5 was compulsory with the exception of patients 38-40 years in whom double blastocyst transfer was performed if no good-quality blastocysts were available. The two co-primary endpoints were ongoing pregnancy rate and ongoing implantation rate in the fresh cycle, defined as at least one intrauterine viable fetus 10-11 weeks after transfer and number of intrauterine viable fetuses 10-11 weeks after transfer divided by number of blastocysts transferred, respectively. The trial demonstrated that REKOVELLE was at least as effective as follitropin alfa in terms of ongoing pregnancy rate and ongoing implantation rate, as shown in Table 3. Table 3 Ongoing pregnancy rate and ongoing implantation rate in ESTHER-1 trial REKOVELLE in an Follitropin alfa Difference [95% CI] individualised dosing regimen (N=665) (N=661) Ongoing pregnancy rate 30.7% 31.6% -0.9% [-5.9%; 4.1%] Ongoing implantation rate 35.2% 35.8% -0.6% [-6.1%; 4.8%] Population: all randomised and exposed The impact of the AMH-based dosing regimen of REKOVELLE was also assessed in secondary endpoints, such as ovarian response and OHSS risk management. In the overall trial population, the mean number of oocytes retrieved was 10.0 ± 5.6 with REKOVELLE (N=636) in the individualised dosing regimen and 10.4 ± 6.5 with follitropin alfa (N=643) at a starting dose of 150 IU followed by dose adjustments. Among patients with AMH ≥15 pmol/L, the ovarian response with REKOVELLE (N=355) and follitropin alfa (N=353), respectively, was as follows: mean number of oocytes retrieved 11.6 ± 5.9 and 13.3 ± 6.9, and proportion of patients with ≥20 oocytes 10.1% (36/355) and 15.6% (55/353). In ovulatory patients with polycystic ovaries, the incidence of early moderate/severe OHSS and/or preventive interventions for early OHSS was 7.7% with REKOVELLE and 26.7% with follitropin alfa. Safety – immunogenicity Anti-FSH antibodies were measured pre-dosing and post-dosing in patients undergoing up to three repeated treatment cycles with REKOVELLE (665 patients in cycle 1 in the ESTHER-1 trial as well as 252 patients in cycle 2 and 95 patients in cycle 3 in the ESTHER-2 trial). The incidence of anti-FSH antibodies after treatment with REKOVELLE was 1.1% in cycle 1, 0.8% in cycle 2 and 1.1% in cycle 3. These rates were similar to the incidence of pre-existing anti-FSH antibodies before exposure to REKOVELLE in cycle 1 which was 1.4%, and comparable to the incidences of anti-FSH antibodies after treatment with follitropin alfa. In all patients with anti-FSH antibodies, titres were undetectable or very low and without neutralising capacity. Repeated treatment with REKOVELLE of patients with pre-existing or treatment-induced anti-FSH antibodies did not increase the antibody titre, was not associated with decreased ovarian response, and did not induce immune-related adverse events. There is no clinical trial experience with REKOVELLE in the long GnRH agonist protocol.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties The pharmacokinetic profile of follitropin delta has been investigated in healthy female subjects and in IVF/ICSI patients undergoing COS. Following repeated daily subcutaneous administrations, REKOVELLE reaches steady-state within 6 to 7 days with a threefold higher concentration compared with the concentration after the first dose. Circulating levels of follitropin delta are inversely related to the body weight, which supports individualised dosing based on body weight. Follitropin delta leads to greater exposure than follitropin alfa. Absorption After daily subcutaneous administration of REKOVELLE, the time to maximum serum concentration is 10 hours. The absolute bioavailability is about 64%. Distribution The apparent volume of distribution is about 25 L after subcutaneous administration and the volume of distribution at steady state is 9 L after intravenous administration. Within the therapeutic dose range, exposure to follitropin delta increases proportionally with the dose. Elimination Following subcutaneous administration, the apparent clearance of follitropin delta is 0.6 L/h and the clearance after intravenous is 0.3 L/h. The terminal elimination half-life after single subcutaneous administration is 40 hours and after multiple subcutaneous administration is 28 hours. The apparent clearance for follitropin delta is low, i.e. 0.6 L/h after multiple subcutaneous administration, leading to high exposure. Follitropin delta is expected to be eliminated similarly to other follitropins, i.e. mainly by the kidneys. The fraction of follitropin delta excreted unchanged in the urine was estimated to 9%.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
