Quest for the right Drug
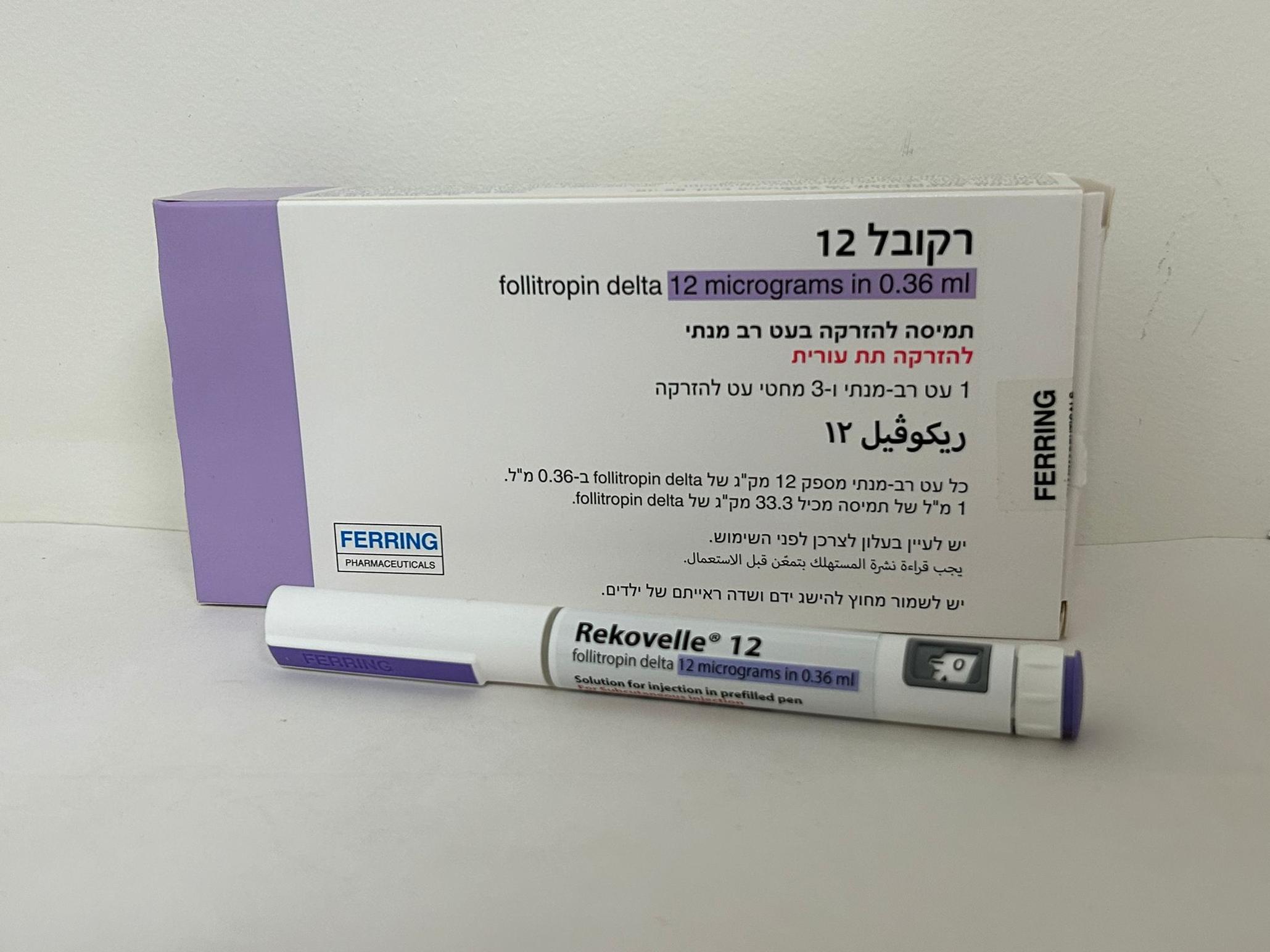
רקובל REKOVELLE (FOLLITROPIN DELTA)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Treatment should be initiated under the supervision of a physician experienced in the treatment of fertility problems. Posology The posology of REKOVELLE is individualised for each patient and aims to obtain an ovarian response which is associated with a favourable safety/efficacy profile, i.e. aims to achieve an adequate number of oocytes retrieved and reduce the interventions to prevent ovarian hyperstimulation syndrome (OHSS). REKOVELLE is dosed in micrograms (see section 5.1). The dosing regimen is specific for REKOVELLE and the microgram dose cannot be applied to other gonadotropins. For the first treatment cycle, the individual daily dose will be determined on the basis of the woman’s serum anti-Müllerian hormone (AMH) concentration and her body weight. The dose should be based on a recent determination of AMH (i.e. within the last 12 months) measured by the following diagnostic tests: ELECSYS AMH Plus immunoassay from Roche (i.e. assay used in clinical development trials), or alternatively the ACCESS AMH Advanced from Beckman Coulter (see section 4.4). The individual daily dose is to be maintained throughout the stimulation period. For women with AMH <15 pmol/L the daily dose is 12 micrograms, irrespective of body weight. For women with AMH ≥15 pmol/L the daily dose decreases from 0.19 to 0.10 micrograms/kg by increasing AMH concentration (Table 1). The dose is to be rounded off to the nearest 0.33 micrograms to match the dosing scale on the injection pen. The maximum daily dose for the first treatment cycle is 12 micrograms. For calculation of the REKOVELLE dose, the body weight is to be measured without shoes and overcoat just prior to start of stimulation. Table 1 Dosing regimen AMH (pmol/L) <15 15-16 17 18 19-20 21-22 23-24 25-27 28-32 33-39 ≥40 Fixed daily dose 12 0.19 0.18 0.17 0.16 0.15 0.14 0.13 0.12 0.11 0.10 of REKOVELLE mcg mcg/kg The AMH concentration is to be expressed in pmol/L and is to be rounded off to the nearest integer. If the AMH concentration is in ng/mL, the concentration should be converted to pmol/L by multiplying with 7.14 (ng/mL x 7.14 = pmol/L) before use. mcg: micrograms Potential high responders (patients with AMH >35 pmol/L) have not been studied in a protocol using down-regulation with GnRH agonist. Time of initiating treatment with REKOVELLE depends on the type of protocol. - in a protocol using a gonadotropin-releasing hormone (GnRH) antagonist, the treatment with REKOVELLE should be initiated on day 2 or 3 after start of menstrual bleeding, - in a protocol using down-regulation with a GnRH agonist, the treatment with REKOVELLE should be initiated approximately 2 weeks after the start of agonist treatment Treatment should continue until adequate follicular development (≥3 follicles ≥17 mm) has been achieved, which on average is by the ninth or tenth day of treatment (range 5 to 20 days). With pituitary desensitisation caused by a GnRH agonist, a longer duration of stimulation and therefore a higher total dose of REKOVELLE may be necessary to achieve adequate follicular response. A single injection of 250 micrograms recombinant human chorionic gonadotropin (hCG) or 5,000 IU hCG is administered to induce final follicular maturation. In patients with excessive follicular development (of ≥25 follicles ≥12 mm), treatment with REKOVELLE should be stopped and triggering of final follicular maturation with hCG should not be performed. For subsequent treatment cycles, the daily dose of REKOVELLE should be maintained or modified according to the patient’s ovarian response in the previous cycle. If the patient had adequate ovarian response in the previous cycle without developing OHSS, the same daily dose should be used. In case of ovarian hypo-response in the previous cycle, the daily dose in the subsequent cycle should be increased by 25% or 50%, according to the extent of response observed. In case of ovarian hyper- response in the previous cycle, the daily dose in the subsequent cycle should be decreased by 20% or 33%, according to the extent of response observed. In patients who developed OHSS or were at risk of OHSS in a previous cycle, the daily dose for the subsequent cycle is 33% lower than the dose used in the cycle where OHSS or risk of OHSS occurred. The maximum daily dose is 24 micrograms. Elderly There is no relevant use of REKOVELLE in the elderly population. Patients with renal and hepatic impairment Safety, efficacy and pharmacokinetics of REKOVELLE in patients with renal or hepatic impairment have not been specifically studied in clinical trials. Although limited, data did not indicate a need for a different dosing regimen of REKOVELLE in this patient population (see section 4.4). Polycystic ovarian syndrome patients with anovulatory disorders Anovulatory patients with polycystic ovarian syndrome have not been studied. Ovulatory patients with polycystic ovaries have been included in clinical trials (see section 5.1). Paediatric population There is no relevant use of REKOVELLE in the paediatric population. Method of administration REKOVELLE is intended for subcutaneous use, preferably in the abdominal wall. The first injection should be performed under direct medical supervision. Patients must be educated on how to use the REKOVELLE injection pen and to perform injections. Self-administration should only be performed by patients who are well motivated, adequately trained and have access to expert advice. For instructions on the administration with the pre-filled pen, see the ”Instructions for Use”.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
